Figure 1.
c-Src Phosphorylates Human TIMP-2 In Vitro and In Vivo
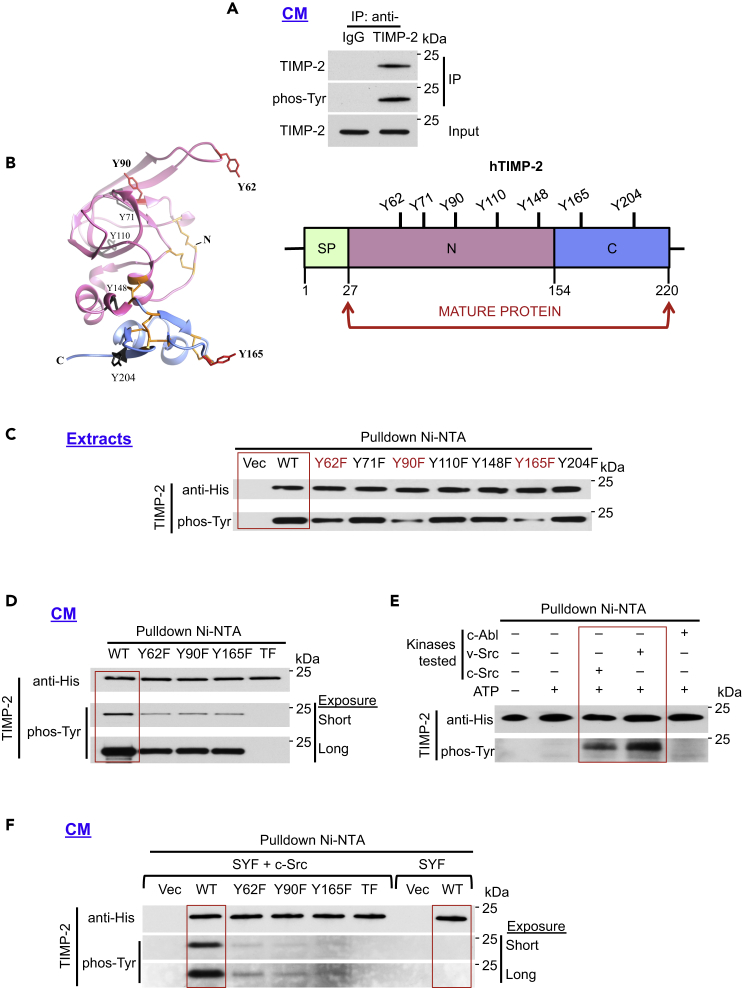
(A) Endogenous secreted TIMP-2 was immunoprecipitated from 10X concentrated HT1080 cell-conditioned media (CM) using anti-TIMP-2 or IgG (control) and analyzed by immunoblotting for phosphorylation using an anti-pan-phosphotyrosine antibody (phos-Tyr, 4G10).
(B) 3D (PDB: 1BR9) and linear domain structures of human TIMP-2 (hTIMP-2). All seven TIMP-2 tyrosine residues (Y) (black) are shown. Numbering is based on the full-length protein sequence (aa 1–220).
(C and D) (C) TIMP-2 His6-tagged wild type (WT), vector control (Vec), and individual mutant plasmids were transiently expressed in HEK293H cells and pulled down from cell extracts (D) or CM and immunoblotted with indicated antibodies to assess phosphorylation.
(E) Recombinant (rTIMP-2-His6) was used as the substrate in an in vitro kinase assay in the presence of full-length c-Src, v-Src, or c-Abl tyrosine kinases. Following pulldown, immunoblotting was performed to assess tyrosine phosphorylation using phos-Tyr, 4G10 antibody.
(F) TIMP-2 constructs were transiently expressed in SYF and SYF + c-Src cells, pulled down from 10X concentrated CM and immunoblotted to determine TIMP-2 tyrosine phosphorylation.
See also Figure S1.