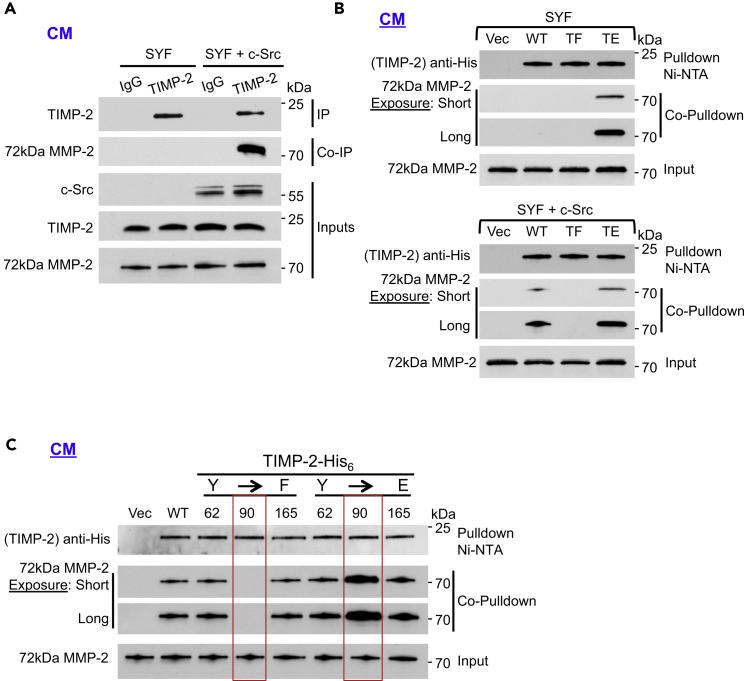
Figure 3.
Phosphorylation of Y90 Is Essential for TIMP-2:proMMP-2 Interaction
(A) SYF and SYF + c-Src cells were serum starved for 18 hr. Immunoprecipitation (IP) of endogenous TIMP-2 from the cell CM was followed by co-immunoprecipitation (co-IP) of 72 kDa proMMP-2 to determine interaction.
(B) SYF (top) and SYF + c-Src (bottom) were transiently transfected with Vec control, WT TIMP-2-His6, and mutants (TF and TE). Pulldown was performed from the CM followed by immunoblotting and co-pulldown to assess protein interaction.
(C) HEK293H cells were transiently transfected with the indicated plasmids. Vec control, WT, and non-phosphorylatable (F) and phosphomimetic (E) TIMP-2 mutants were pulled down from the CM. Interaction of TIMP-2 proteins with secreted 72 kDa proMMP-2 was determined by co-pulldown and immunoblotting.
See also Figure S3.