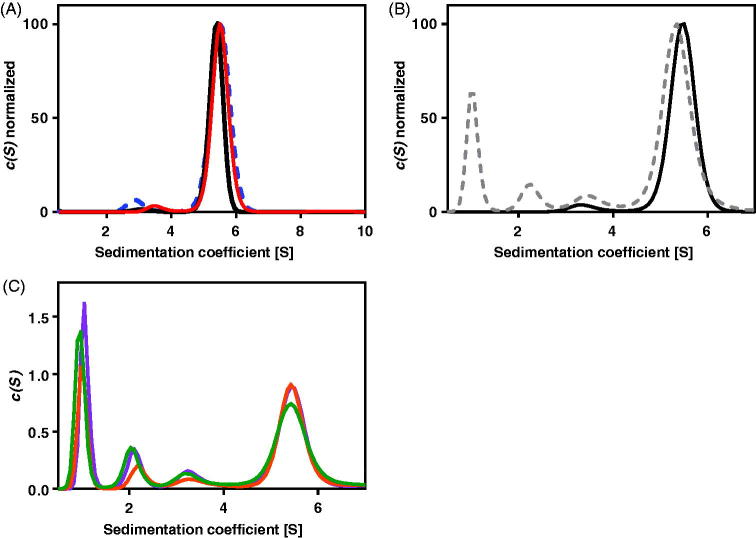
Figure 2.
The absorption at 280 nm, A280nm(s), and the interference(s) profiles obtained from fitting the continuous c(s) distribution model to the sedimentation velocity data acquired for Helicobacter pylori AdSS at 20 mM HEPES-NaOH pH 6.8 + 1 mM TCEP, at 20 °C. (A) A280 nm(s) profile for the 4.4 µM of the apo AdSS (black); 4.4 µM AdSS in a binary complex with 0.1 mM IMP (red); and 3.3 µM AdSS in a ternary complex with 0.1 mM IMP and 1 mM hadacidin (blue dashed line) (data normalised). (B) A280 nm(s) (black) and interference(s) (grey dashed line) profiles for the apo enzyme, 5.1 µM (data normalised). (C) Interference(s) profiles for the 5.1 µM AdSS in a binary complex with 3.0 mM IMP (orange); ternary complex with 3 mM IMP and 6 mM GDP, in the presence of 3.3 mM MgSO4 (violet); and quaternary complex with 3.0 mM IMP, 6.0 mM GDP and 5.5 mM hadacidin, in the presence of 3.3 mM MgSO4 (green). Two species with sedimentation coefficients of about 1S and 2S, corresponding to a molecular mass of about 10 and 30 kDa, as observed only in the interference profiles, not in the A280nm profiles (see data on panel B), are therefore probably non-protein contaminants, which is additionally confirmed by the single band observed on the SDS electrophoresis gel (see Figure 1).