Figure 1. Switching between interphase and mitosis.
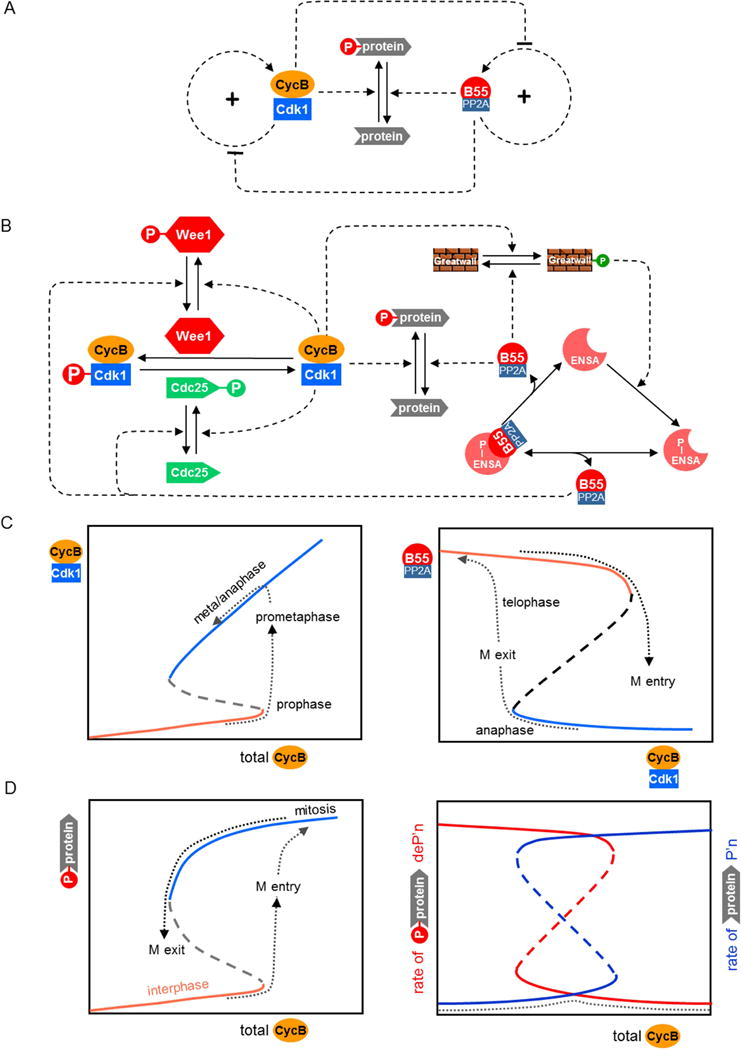
(A) Regulatory motif. Many cellular proteins are phosphorylated during mitosis by Cyclin B-dependent protein kinase-1 (CycB:Cdk1). During interphase most of these proteins are unphosphorylated, due to the action of protein phosphatases, such as B55:PP2A. Both CycB:Cdk1 and B55:PP2A are activated by positive feedback loops that create two separate bistable switches. The two switches are mutually inhibitory, so that, if the kinase switch is ON, then the phosphatase switch is OFF, and vice versa. (B) Detailed biochemical mechanism. CycB:Cdk1 is inhibited by phosphorylation of the kinase subunit by Wee1 and activated by dephosphorylation by Cdc25. Both Wee1 and Cdc25 are phosphorylated by CycB:Cdk1; an inhibitory phosphorylation in the first case and activatory in the second [39]. These redundant positive feedback loops create a bistable toggle switch for CycB:Cdk1 activity [4]. On the other side, B55:PP2A is inactivated by binding to phosphorylated ENSA [40,41]. B55:PP2A can activate itself by dephosphorylating P-ENSA [42], but this action is opposed by a kinase, Greatwall in its phosphorylated form. This motif, called a ‘feedback-amplified domineering substrate’, creates a bistable toggle switch for B55:PP2A activity [43]. The two toggle switches are mutually inhibitory, because CycB:Cdk1 is the kinase that phosphorylates Greatwall, and B55:PP2A is the phosphatase that dephosphorylates Wee1 and Cdc25 [7,12]. (C) Signal-response curves. (Left panel) Steady state activity of CycB:Cdk1 as a function of total Cyclin B in a cell entering mitosis and proceeding into anaphase. (Right panel) Steady state activity of B55:PP2A as a function of CycB:Cdk1 activity in a cell. B55:PP2A activity switches OFF as a cell enters mitosis and switches ON as a cell exits mitosis. (D) Substrate phosphorylation during interphase and mitosis. Total concentration of B-type Cyclins increases during interphase and early mitosis, and decreases as cells exit mitosis and return to interphase. (Left panel) Cdk-targeted substrates are heavily phosphorylated in mitosis and mostly unphosphorylated in interphase. (Right panel) During interphase the rate of substrate phosphorylation is low and dephosphorylation is high, and vice versa during mitosis. Consequently, the rate of futile cycling (dashed line) is low throughout the cell cycle, because CDK and CAP are never active simultaneously.
