Figure 3. Controlling the transition from prometaphase into anaphase.
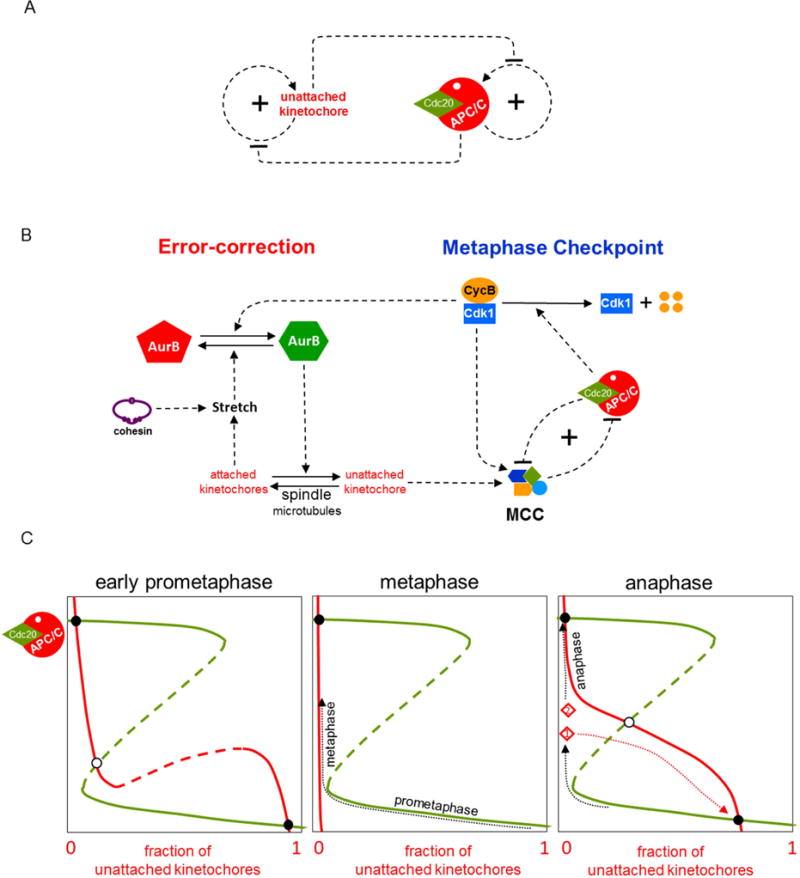
(A) Regulatory motif. Mitotic cells have a surveillance mechanism for detecting and correcting unattached kinetochores (uKTs). Positive feedback in this mechanism creates a bistable switch that allows replicated chromosomes to accumulate in a bioriented state (with sister KTs stretched by their attachment to opposite poles of the mitotic spindle). Meanwhile, uKTs send an inhibitory signal to the ‘anaphase promoting complex/cyclosome’ (APC/C), whose activity is also governed by a bistable switch. When the APC/C is activated, it silences a component in the uKT positive feedback loop [7,45]. (B) Detailed influence diagram. (Left side) The error-correction module. If a pair of sister chromatids is properly attached to the mitotic spindle at both kinetochores (i.e., bioriented), then the attached kinetochores (aKT) become stretched, which blocks the action of Aurora B (AurB) kinase. If a pair of uKTs is improperly attached by only a single kinetochore, then active AurB at that centromere induces detachment of microtubules (MTs) from the aKT. This error-correcting action of AurB allows the replicated chromosome to try again to achieve biorientation on the mitotic spindle. The mutual antagonism between aKT and AurB creates a bistable switch that maintains a population of uKTs until all the cell’s chromosomes are properly aligned on the spindle. (Right side) The metaphase checkpoint. uKTs promote assembly of a mitotic checkpoint complex (MCC), which binds to and inhibits the APC/C, thereby blocking progression of the cell into anaphase. Active APC/C, on the other hand, promotes disassembly of the MCC; but this double-negative feedback loop is insufficient, in its own right, to create a bistable switch. Bistability requires that active APC/C promotes the degradation of CycB; thereby destroying CycB:Cdk1 activity, which is necessary for assembling the MCC [46]. The entire motif (on the right side of the diagram) is called a ‘feedback-amplified domineering substrate’, and the conditions for bistability are described in Ref [43]. Experimental evidence indicates that the metaphase checkpoint is indeed a bistable toggle switch [45,47]. In addition, CycB:Cdk1 activity feeds back to the error-correction module as a co-activator of AurB. (C) The dynamics of mitotic progression. (Left panel) Green curve: steady state activity of APC/C as a function of the fraction of uKTs; red curve: steady state fraction of uKTs as a function of APC/C activity. During prometaphase, the control system persists at the stable steady state with low APC/C activity (the black circle in the lower-right corner), with many chromosomes in the 00-state of KT attachment and the APC/C inactive. As chromosomes become bioriented (11-state), the red curve moves steadily to the left. (middle panel) When all the chromosomes are properly attached to the mitotic spindle (metaphase), the red curve is pressed against the vertical axis, and the cell lifts the metaphase checkpoint by activating APC/C. (There is now a single stable steady state, the black circle in the upper-left corner.) (Right panel) As the cell proceeds through metaphase, if some aKTs become unattached (either spontaneously or by experimental manipulation), an error-signal recreates the prometaphase steady state (the APC/C inactive state in the lower-right corner) and a cell that has not yet passed the ‘point-of-no-return’ will return to prometaphase (the red trajectory from point 1). Cleavage of cohesins during anaphase eliminates kinetochore stretch and recreates the APC/C inactive steady state, but anaphase cells (point 2 in the panel) are beyond the ‘point-of-no-return’ and so they maintain their progression toward the upper-left steady state, where APC/C is fully active.
