Abstract
Metallotriazine complexes possess interesting biological and medicinal properties, and the present study focuses on the synthesis, characterization, and antimicrobial activity of four novel copper-triazine derivatives in search of potent antibacterial and antifungal drug leads. In this study, 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-4,4′-disulfonic acid monosodium salt (L1, ferrozine) and 3-(2-pyridyl)-5,6-di(2-furyl)-1,2,4-triazine-5,5′-disulfonic acid disodium salt (L2, ferene) have been used as ligands to study the complexation towards copper(II). The synthesized complexes, [CuCl2(ferrozine)]·7H2O·MeOH (1), [CuCl2(ferrozine)2]·5H2O·MeOH (2), [CuCl2(ferene)]·H2O·MeOH (3), and [CuCl2(ferene)2]·H2O·MeOH (4), have been characterized spectroscopically, and preliminary bioassays have been carried out. FTIR spectroscopic data have shown that N=N and C=N stretching frequencies of complexes have been shifted towards lower frequencies in comparison with that of the ligands, confirming new bond formation between Cu and N, which in turn lowers the strength of N=N and C=N bonds. In addition, a bathochromic shift has been observed for UV-visible spectra of complexes (1), (2), (3), and (4). Furthermore, elemental analysis data have been useful to obtain empirical formulas of these complexes and to establish the purity of each complex. Complexes (1) and (2) have shown antibacterial activity for both S. aureus (ATCC® 25923) and E. coli (ATCC® 25922) at 1 mg/disc concentration, and ferrozine has shown a larger inhibition zone against the clinical sample of C. albicans at 1 mg/disc concentration in comparison with the positive control, fluconazole.
1. Introduction
Transition metals have numerous and unique biological, chemical, and physical properties due to the availability of d electrons in valance shells. Much attention has been focused on copper complexes due to their various potential biological activities [1–4] out of which antimicrobial [5] and antiviral activities is paramount [6–15].
Since triazine is a well-known natural material which possesses many biological properties [16–21], it is not surprising that organometallic complexes of triazine with first row transition metals (Mn [22, 23], Co [24, 25], Ni [24, 25], Cu [22, 24–28], and Zn [25]), with second row transition metals (Ru [29], Pd [30], Ag [31], and Cd [32]), and with third row transition metals (Re [33] and Pt [34–36]) have been synthesized, and their activities explored as catalysts [37] and biological agents such as antibacterial [25], anticancer [29, 36], antifouling [24], antifungal [33], anti-HIV [35], antimicrobial [25], antiproliferative [26, 34], antiviral [28, 35], and DNA binding [26, 29, 30] agents.
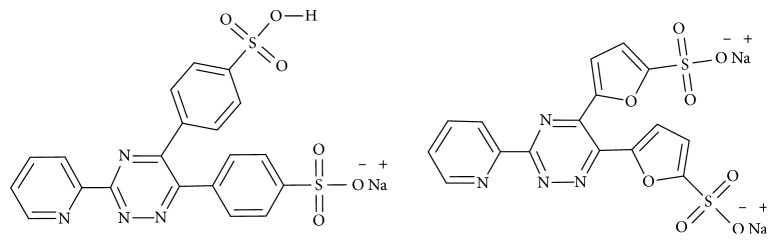
Even though many reports exist of metal complexes of triazine derivatives as detailed above, metal complexes containing the pyridyl-1,2,4-triazine core are relatively unexplored. Platinum(II) complexes of sulphonated 2-pyridyl-1,2,4-triazine have been reported to possess anti-HIV activity [35]. A copper(II) complex bearing 2,4,6-tris(2-pyridyl)-1,3,5-triazine ligand has been reported to bind DNA in a moderately strong way exhibiting significantly better anticancer activity against breast cancer in comparison with cisplatin [26]. An octahedral complex of rhenium(V), ML1L2L3L4 (where L1 = oxo, L2 = chloride, L3 = triphenylphosphine, and L4 = 3-hydrazino-5,6-diphenyl-1,2,4-triazine), has shown comparable antifungal activity against Alternaria alternata and Aspergillus niger [33]. We ourselves have explored the potential of using rhenium complexes of ferene and ferrozine (Figure 1) as biological imaging agents [38]. In our most recent work, we have commented on the possible use of the scaffold of sulfonated pyridyl triazine complexes being utilized as serum albumin transporters [39]. As such, it seems prudent to now explore its binding towards copper.
Figure 1.
Structure of 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-p,p′-disulfonic acid monosodium salt (L1) (a) and 3-(2-pyridyl)-5,6-di(2-furyl)-1,2,4-triazine-5,5′-disulfonic acid disodium salt (L2) (b).
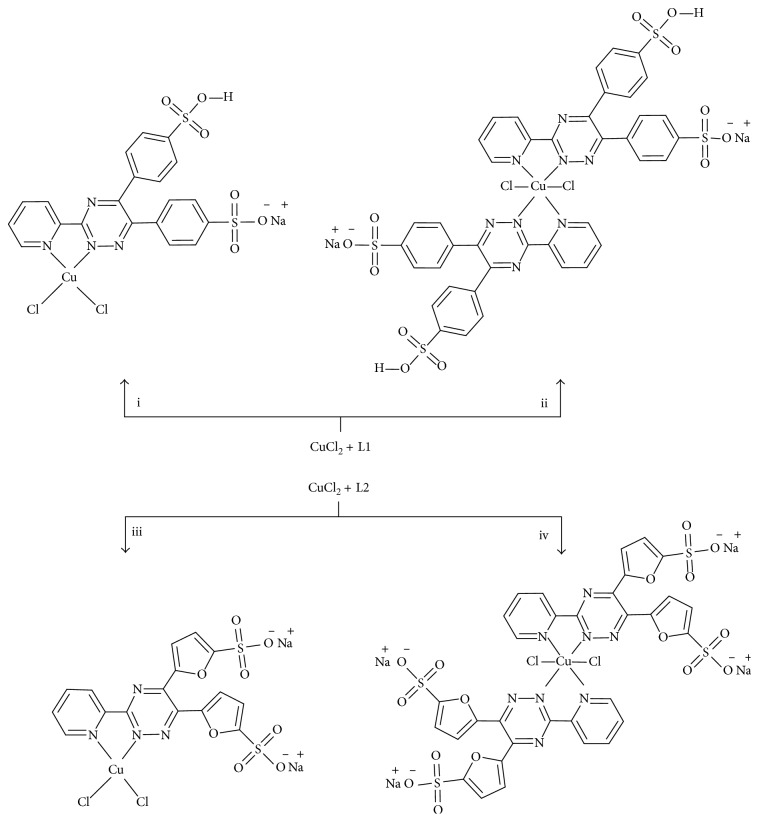
Thus, the current study explores the synthesis of four novel water-soluble complexes of the type, MLnCl2 (Figure 2) (where M = Cu2+, L = 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-4′,4″-disulfonic acid sodium salt/3-(2-pyridyl)-5,6-di(2-furyl)-1,2,4-triazine-5′,5″-disulfonic acid disodium salt, and n = 1/2), their chemical characterization, and preliminary tests to assess antimicrobial activity of above synthesized complexes as well as of the ligands.
Figure 2.
Synthetic routes for ML1Cl2 (complex (1)) (i), M(L1)2Cl2 (complex (2)) (ii), ML2Cl2 (complex (3)) (iii), and M(L2)2Cl2 (complex (4)) (iv) complexes. NB: L1 = 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-p,p′-disulfonic acid monosodium salt; L2 = 3-(2-pyridyl)-5,6-di(2-furyl)-1,2,4-triazine-5,5′-disulfonic acid disodium salt. Solvent molecules in complexes (1)–(4) have been omitted for clarity. Molar ratios of reactants: (i) CuCl2 : L1 = 1 : 1, (ii) CuCl2 : L1 = 1 : 2, (iii) CuCl2 : L2 = 1 : 1, and (iv) CuCl2 : L2 = 1 : 2.
2. Experimental
2.1. Materials Used
All chemicals and reagents used for the synthesis were commercially available and used without further purification. 3-(2-Pyridyl)-5,6-diphenyl-1,2,4-triazine-4,4′-disulfonic acid monosodium salt (ferrozine), 3-(2-pyridyl)-5,6-di(2-furyl)-1,2,4-triazine-5,5′-disulfonic acid disodium salt (ferene), and methanol ACS reagent (assay ≥99.8%) were purchased from Sigma-Aldrich, and copper(II) chloride dihydrate was purchased from Research-Lab Fine Chem Industries. Mueller-Hinton agar was purchased from Hardy Diagnostics, USA. Sodium chloride, sodium hydroxide, and dextrose were purchased from HiMedia Laboratories. The bacteria were obtained by the Industrial Technology Institute, Colombo.
2.2. Instrumentation
Elemental analysis was carried out on PerkinElmer 2400 Series II CHNS/O Elemental Analyzer at Atlantic Microlabs, USA. IR spectra were recorded using Thermo Scientific NICOLET iS10 spectrophotometer in the spectral range 4000–650 cm−1 for both ligands and complexes. Thermo Spectronic Helios alpha UV-Vis double-beam spectrophotometer was used to measure the absorbance in the range of 190–1100 nm, and baseline correction was performed using matched quartz cuvettes. High-resolution mass spectra were recorded on an Agilent 6210 ESI TOF LCMS mass spectrometer.
2.3. Synthesis
2.3.1. Preparation of [CuCl2(ferrozine)]·7H2O·MeOH (1)
A solution of ferrozine (0.25 mmol, 0.1269 g) in methanol (8.0 cm3) was added to copper chloride dihydrate (0.25 mmol, 0.0435 g) in methanol (2.0 cm3). Then the resulting mixture was stirred for 2 hours at room temperature and progression of reaction checked using TLC. A light green colour crystalline precipitate was obtained after 2 days and collected by filtration (yield: 0.1264 g, 64%). IR (ATR; ν/cm−1): 1596.84(m) and 1498.22(s), ν C=N and ν N=N. UV-Vis (MeOH; λ max [nm]): 205, 242, 298, and 327. Anal. Calc. for C20H13Cl2CuN4NaO6S2·7H2O·CH3OH: C, 32.12; H, 3.98; N, 7.14. Found: C: 31.68%, H: 3.80%, and N: 7.42%. ESI-MS (m/z): [M − H]− calcd for C20H13ClCuN4O6S2, 565.9179; found, 565.9188.
2.3.2. Preparation of [CuCl2(ferrozine)2]·5H2O·MeOH (2)
A procedure similar to that given above was followed using copper chloride dihydrate (0.25 mmol, 0.0435 g) and ferrozine (0.50 mmol, 0.2538 g). The resulting mixture was stirred for 5 hours. A dark green colour crystalline precipitate was obtained after 2 days and collected by filtration (yield: 0.1937 g, 62%). IR (ATR; ν/cm−1): 1595.69(m) and 1498.50(s), ν C=N and ν N=N. UV-Vis (MeOH; λ max [nm]): 213, 240, 301, and 334. Anal. Calc. for C40H26Cl2CuN8Na2O12S4·5H2O·CH3OH: C, 39.66; H, 3.25; N, 9.03. Found: C: 39.29%, H: 3.76%, N: 9.23%. ESI-MS (m/z): [M − H]− calcd for C40H26CuN8O12S4, 999.9833; found, 999.9776.
2.3.3. Preparation of [CuCl2(ferene)]·H2O·MeOH (3)
A solution of ferene (0.25 mmol, 0.1236 g) in methanol (8.0 cm3) was added to copper chloride dihydrate (0.25 mmol, 0.0435 g) in methanol (2.0 cm3). Then the resulting mixture was stirred for 6 hours at room temperature and progression of reaction checked using TLC technique initially and at the end. A yellow colour crystalline precipitate was obtained after 1 day and collected by filtration (yield: 0.1183 g, 75%). IR (ATR; ν/cm−1): 1567.49(m) and 1499.15(s), ν C=N and ν N=N. UV-Vis (MeOH; λ max [nm]): 202, 239, 338, and 371. Anal. Calc. for C16H8Cl2CuN4O8S2·H2O·CH3OH: C, 32.16; H, 2.54; N, 8.82. Found: C: 32.12%, H: 2.76%, N: 9.29%. ESI-MS (m/z): [M]− calcd for C16H8CuN4O8S2, 510.9085; found, 510.9084.
2.3.4. Preparation of [CuCl2(ferene)2]·H2O·MeOH (4)
A procedure similar to above was followed using copper chloride dihydrate (0.25 mmol, 0.0435 g) and ferene (0.50 mmol, 0.2472 g). The resulting mixture was stirred for 5 hours. A brown-yellow colour crystalline precipitate was obtained after 1 day and collected by filtration (yield: 0.1912 g, 65%). IR (ATR; ν/cm−1): 1569.82(m) and 1494.40(s), ν C=N and ν N=N. UV-Vis (MeOH; λ max [nm]): 208, 246, 338 and 371. Anal. Calc. for C32H16Cl2CuN8Na4O16S4·H2O·CH3OH: C, 33.78; H, 1.89; N, 9.56. Found: C: 33.76%, H: 2.42%, N: 9.58%.
2.4. Antimicrobial Assay
Compounds were tested against Gram-positive Staphylococcus aureus ATCC® 25923 and Gram-negative Escherichia coli ATCC® 25922 bacterial species and a clinical isolate of Candida albicans as a fungal species. Antimicrobial assay was performed by a standard disk diffusion assay [40] where the inhibition zones were measured and expressed as a mean of three replicates. Gentamycin and flucanazole were used as positive controls, and methanol was used as the negative control.
3. Results and Discussion
3.1. Synthesis
Copper chloride and the relevant ligands were used in 1 : 1 and 1 : 2 ratios to synthesize the desired metal complexes (Figure 2). Thin-layer chromatography (TLC) was initially used to monitor the progress of reaction, and visualization of spots was done using an iodine bath.
3.2. FTIR Analysis
FTIR data were recorded for dried crystals of ligands and complexes (1)–(4), and literature values were utilized where relevant [41]. The stretching frequency of the pyridine ring (ν C=N) and stretching frequency of the triazine ring (ν N=N) are considered mostly, because their values change upon formation of new bonds serving as good indicators of complex formation.
Stretching frequencies of N=N and C=N in complexes (1) and (2) have shifted to lower frequencies as expected, compared to those values of the free ferrozine ligand, due to σ donation of N lone pair which lowers strength of N=N and C=N bonds (Table 1). Furthermore, a broad band around 3400–3300 cm−1 was observed due to OH groups from methanol or water.
Table 1.
FTIR data comparison chart of complexes (1)–(4) in comparison with those of free ligands.
| ν C=N (cm−1) | ν N=N (cm−1) | |
|---|---|---|
| Ferrozine | 1608 | 1503 |
| Complex (1) | 1596 | 1498 |
| Complex (2) | 1595 | 1498 |
| Ferene | 1589 | 1507 |
| Complex (3) | 1567 | 1499 |
| Complex (4) | 1570 | 1494 |
Similarly, stretching frequencies of N=N and C=N in complexes (3) and (4) were observed at lower frequencies in comparison with those of the free ferrozine ligand (Table 1), and a broad band was observed around 3400–3300 cm−1 due to OH groups of solvent.
3.3. UV-Visible Spectroscopy
UV-Vis spectra of reactants and complexes (1, 2, 3, and 4) were recorded in methanol at room temperature (Figure 3, Table S1, Supplementary Materials). The absorption wavelengths of complexes (1)–(4) have shifted towards longer wavelengths (bathochromic shift) compared to the wavelengths of the reactants (copper, ferrozine, and ferene). Both ferrozine and ferene have aromatic ring systems, and π–π ∗ transitions are thus possible [42]. These results are in agreement with those previously reported for zinc complexes of ferene and ferrozine [39] where a bathochromic shift was observed for both mono and bis complexes in comparison with that of the free ligand.
Figure 3.
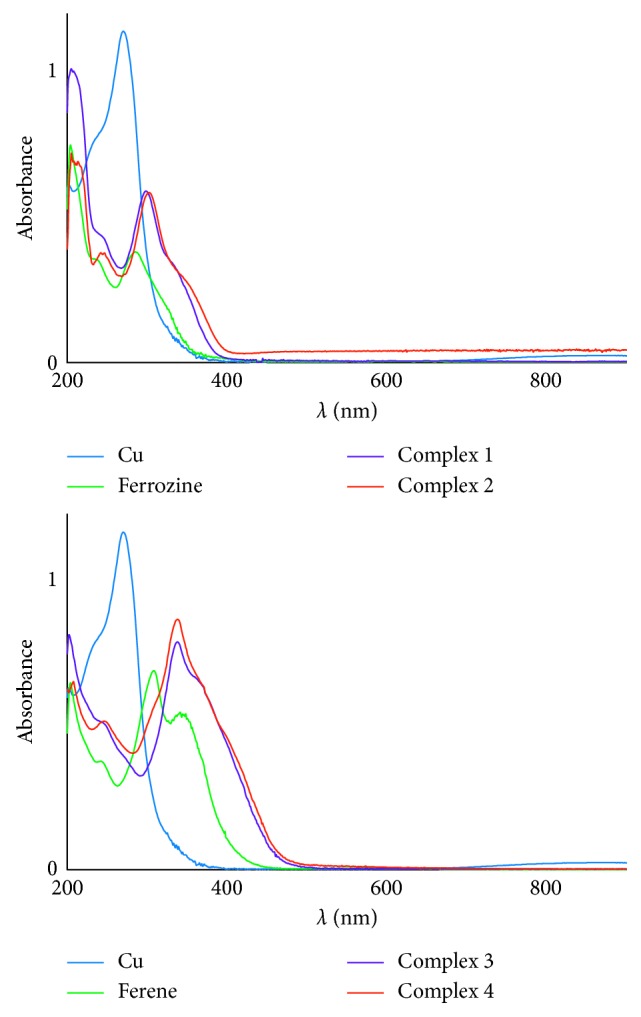
UV-visible spectra recorded in methanol of ferrozine, complexes (1) and (2) (a) and ferene, complexes (3) and (4) (b).
3.4. Elemental Analysis
Empirical formulas related to experimental values aided in obtaining the exact molecular formulas of all four complexes (Table 2). It can be seen that experimental values are within ±0.4% of expected values indicating purity of the synthesized complexes.
Table 2.
Elemental analysis data of complexes.
| Complex | Value | C (%) | H (%) | N (%) |
|---|---|---|---|---|
| (1) | Calculated | 32.12 | 3.98 | 7.14 |
| Experimental | 31.68 | 3.80 | 7.42 | |
|
| ||||
| (2) | Calculated | 39.66 | 3.25 | 9.03 |
| Experimental | 39.29 | 3.76 | 9.23 | |
|
| ||||
| (3) | Calculated | 32.16 | 2.54 | 8.82 |
| Experimental | 32.12 | 2.76 | 9.29 | |
|
| ||||
| (4) | Calculated | 33.78 | 1.89 | 9.55 |
| Experimental | 33.76 | 2.42 | 9.58 | |
3.5. Antimicrobial Activity
All four complexes and ligands were studied in vitro for their antimicrobial activity against Gram-positive Staphylococcus aureus ATCC® 25923 and negative bacteria Escherichia coli ATCC® 25922 as well as the unicellular fungal species, Candida albicans. Inhibition zones were obtained by adding a concentration of 1 mg/disc, and the diameters of the zones are given in Table 3 for bacteria and Table 4 for fungi.
Table 3.
Mean inhibition zone diameter at 1 mg/disc of complexes (1) and (2) and at 20 μg/disc of gentamicin.
| Mean inhibition zone diameter ± SEM (mm) | ||
|---|---|---|
| S. aureus ATCC® 25923 | E. coli ATCC® 25922 | |
| Complex (1) | 8.75 ± 0.75 | 7.50 ± 1.00 |
| Complex (2) | 7.00 ± 0.00 | 7.75 ± 0.25 |
| Positive control (gentamicin) | 26.00 ± 1.50 | 30.75 ± 0.75 |
| Negative control | ND | ND |
ND, not detected.
Table 4.
Mean inhibition zone diameter for Candida albicans at 1 mg/disc of ferrozine and at 1 mg/disc of fluconazole.
| Mean inhibition zone diameter ± SEM (mm) | |
|---|---|
| Ferrozine | 13.00 ± 2.00 |
| Fluconazole | 29.75 ± 0.25 |
Analysis of the inhibition zone diameter revealed that only complex (1) and complex (2) show moderate antibacterial activity when compared to the positive control. It is interesting to see that ferrozine ligand demonstrates antifungal activity.
Antimicrobial activity reported here is of moderate value. Further studies are warranted to optimize this system for greater activity.
4. Conclusions
We have described the synthesis of four novel water-soluble copper complexes bearing sulfonated pyridyl triazine ligands. FTIR spectroscopic data have confirmed the existence of Cu-N bonds in all four complexes because stretching frequencies of N=N and C=N complexes have been shifted towards lower frequencies in comparison with that of the ligands. In UV-Vis spectra, a bathochromic shift has been observed for complexes (1)–(4). Furthermore, elemental analysis data have been useful to obtain empirical formulas of these complexes and to establish the purity of each complex.
Preliminary bioassays in antimicrobial activity showed moderate antibacterial activity with complexes (1) and (2) whereas ferrozine showed antifungal activity against Candida albicans. To the best of our knowledge, we are the first to report on the antifungal activity of ferrozine. These findings provide a potential lead for antimicrobial drug development.
Acknowledgments
Financial assistance from the University of Sri Jayewardenepura (Grant no. ASP/01/RE/SCI/2015/19) is gratefully acknowledged. Clinical sample of Candida albicans was provided by the Department of Microbiology, Faculty of Medicine, University of Colombo. An earlier version of this work with two of the complexes detailed here was presented as an abstract at Chemistry in Sri Lanka 2015 as found in the link http://www.ichemc.edu.lk/wp-content/uploads/2015/10/vol-31-no.-2.pdf. The authors also acknowledge the Centre for Advanced Material Research of the University of Sri Jayewardenepura.
Data Availability
The data used to support the findings of this study are included within the article and within the Supplentary Information file.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Supplementary Materials
Table S1: comparison of UV-Vis data of ferrozine, ferene, and complexes (1)–(4) is presented in a tabulated form in Supplementary Materials.
References
- 1.Valent A., Melník M., Hudecová D., Dudová B., Kivekäs R., Sundberg M. R. Copper(II) salicylideneglycinate complexes as potential antimicrobial agents. Inorganica Chimica Acta. 2002;340:15–20. doi: 10.1016/s0020-1693(02)01062-9. [DOI] [Google Scholar]
- 2.Ibrahim M., Wang F., Lou M. M., et al. Copper as an antibacterial agent for human pathogenic multidrug resistant Burkholderia cepacia complex bacteria. Journal of Bioscience and Bioengineering. 2011;112(6):570–576. doi: 10.1016/j.jbiosc.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 3.Latif Abuhijleh A., Woods C. Synthesis, characterization, and oxidase activities of copper(II) complexes of the anticonvulsant drug valproate. Journal of Inorganic Biochemistry. 1996;64(1):55–67. doi: 10.1016/0162-0134(96)00028-1. [DOI] [Google Scholar]
- 4.Creaven B. S., Duff B., Egan D. A., et al. Anticancer and antifungal activity of copper(II) complexes of quinolin-2(1H)-one-derived Schiff bases. Inorganica Chimica Acta. 2010;363(14):4048–4058. doi: 10.1016/j.ica.2010.08.009. [DOI] [Google Scholar]
- 5.Cervantes H. I., Alvarez J. A., Munoz J. M., Arreguin V., Mosqueda J. L., Macias A. E. Antimicrobial activity of copper against organisms in aqueous solution: a case for copper-based water pipelines in hospitals? American Journal of Infection Control. 2013;41(12):e115–e118. doi: 10.1016/j.ajic.2013.03.309. [DOI] [PubMed] [Google Scholar]
- 6.Shionoiri N., Sato T., Fujimori Y., et al. Investigation of the antiviral properties of copper iodide nanoparticles against feline calicivirus. Journal of Bioscience and Bioengineering. 2012;113(5):580–586. doi: 10.1016/j.jbiosc.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Betanzos-Cabrera G., Ramirez F. J., Munoz J. L., Barron B. L., Maldonado R. Inactivation of HSV-2 by ascorbate-Cu(II) and its protecting evaluation in CF-1 mice against encephalitis. Journal of Virological Methods. 2004;120(2):161–165. doi: 10.1016/j.jviromet.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Sagripanti J. L., Routson L. B., Bonifacino A. C., Lytle C. D. Mechanism of copper-mediated inactivation of herpes simplex virus. Antimicrobial Agents and Chemotherapy. 1997;41(4):812–817. doi: 10.1128/aac.41.4.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White L. A., Freeman C. Y., Forrester B. D., Chappell W. A. In vitro effect of ascorbic acid on infectivity of herpesviruses and paramyxoviruses. Journal of Clinical Microbiology. 1986;24(4):527–531. doi: 10.1128/jcm.24.4.527-531.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borkow G., Zhou S. S., Page T., Gabbay J. A novel anti-influenza copper oxide containing respiratory face mask. PLoS One. 2010;5(6):p. e11295. doi: 10.1371/journal.pone.0011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horie M., Ogawa H., Yoshida Y., et al. Inactivation and morphological changes of avian influenza virus by copper ions. Archives of Virology. 2008;153(8):1467–1472. doi: 10.1007/s00705-008-0154-2. [DOI] [PubMed] [Google Scholar]
- 12.Noyce J. O., Michels H., Keevil C. W. Inactivation of influenza A virus on copper versus stainless steel surfaces. Applied and Environmental Microbiology. 2007;73(8):2748–2750. doi: 10.1128/aem.01139-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieto-Juarez J. I., Pierzchla K., Sienkiewicz A., Kohn T. Inactivation of MS2 coliphage in Fenton and Fenton-like systems: role of transition metals, hydrogen peroxide and sunlight. Environmental Science & Technology. 2010;44(9):3351–3356. doi: 10.1021/es903739f. [DOI] [PubMed] [Google Scholar]
- 14.Yahya M. T., Straub T. M., Gerba C. P. Inactivation of coliphage MS-2 and poliovirus by copper, silver, and chlorine. Canadian Journal of Microbiology. 1992;38(5):430–435. doi: 10.1139/m92-072. [DOI] [PubMed] [Google Scholar]
- 15.Abad F. X., Pinto R. M., Diez J. M., Bosch A. Disinfection of human enteric viruses in water by copper and silver in combination with low levels of chlorine. Applied and Environmental Microbiology. 1994;60(7):2377–2383. doi: 10.1128/aem.60.7.2377-2383.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menicagli R., Samaritani S., Signore G., Vaglini F., Dalla Via L. In vitro cytotoxic activities of 2-alkyl-4,6-diheteroalkyl-1,3,5-triazines: new molecules in anticancer research. Journal of Medicinal Chemistry. 2004;47(19):4649–4652. doi: 10.1021/jm0495374. [DOI] [PubMed] [Google Scholar]
- 17.Melato S., Prosperi D., Coghi P., Basilico N., Monti D. A combinatorial approach to 2,4,6-trisubstituted triazines with potent antimalarial activity: combining conventional synthesis and microwave-assistance. ChemMedChem. 2008;3(6):873–876. doi: 10.1002/cmdc.200700344. [DOI] [PubMed] [Google Scholar]
- 18.Zhou C., Min J., Liu Z., et al. Synthesis and biological evaluation of novel 1,3,5-triazine derivatives as antimicrobial agents. Bioorganic & Medicinal Chemistry Letters. 2008;18(4):1308–1311. doi: 10.1016/j.bmcl.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 19.Srinivas K., Srinivas U., Bhanuprakash K., Harakishore K., Murthy U. S., Rao V. J. Synthesis and antibacterial activity of various substituted s-triazines. European Journal of Medicinal Chemistry. 2006;41(11):1240–1246. doi: 10.1016/j.ejmech.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Baliani A., Bueno G. J., Stewart M. L., et al. Design and synthesis of a series of melamine-based nitroheterocycles with activity against trypanosomatid parasites. Journal of Medicinal Chemistry. 2005;48(17):5570–5579. doi: 10.1021/jm050177+. [DOI] [PubMed] [Google Scholar]
- 21.Xiong Y. Z., Chen F. E., Balzarini J., De Clercq E., Pannecouque C. Non-nucleoside HIV-1 reverse transcriptase inhibitors. Part 11: structural modulations of diaryltriazines with potent anti-HIV activity. European Journal of Medicinal Chemistry. 2008;43(6):1230–1236. doi: 10.1016/j.ejmech.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Małecki J. G., Machura B., Świtlicka A. X-ray studies, spectroscopic characterisation and DFT calculations for Mn(II), Ni(II) and Cu(II) complexes with 5,6-diphenyl-3-(2-pyridyl)-1,2,4-triazine. Structural Chemistry. 2010;22(1):77–87. doi: 10.1007/s11224-010-9686-7. [DOI] [Google Scholar]
- 23.Najafpour M. M., Boghaei D. M., McKee V. Synthesis, characterization, crystal structure and oxygen-evolution activity of a manganese(II) complex with 2,4,6-tris (2-pyridyl)-1,3,5-triazine. Polyhedron. 2010;29(17):3246–3250. doi: 10.1016/j.poly.2010.09.001. [DOI] [Google Scholar]
- 24.Hemaida H. A. E., Dissouky A. A. E., Sadek S. M. M. Potential antifouling agents: copper, cobalt, and nickel complexes of 3-(2-acetyl pyridylidene) hydrazino-5,6-diphenyl-1,2,4-triazine. Egyptian Journal of Aquatic Research. 2005;31:45–56. [Google Scholar]
- 25.Singh K., Kumar Y., Puri P., Sharma C., Aneja K. R. Antimicrobial, spectral and thermal studies of divalent cobalt, nickel, copper and zinc complexes with triazole Schiff bases. Arabian Journal of Chemistry. 2017;10:S978–S987. doi: 10.1016/j.arabjc.2012.12.038. [DOI] [Google Scholar]
- 26.Abdi K., Hadadzadeh H., Salimi M., Simpson J., Khalaji A. D. A mononuclear copper(II) complex based on the polypyridyl ligand 2,4,6-tris(2-pyridyl)-1,3,5-triazine (tptz), [Cu(tptz)2]2+: X-ray crystal structure, DNA binding and in vitro cell cytotoxicity. Polyhedron. 2012;44(1):101–112. doi: 10.1016/j.poly.2012.06.089. [DOI] [Google Scholar]
- 27.Machura B., Świtlicka A., Kruszynski R., Mroziński J., Kłak J., Kusz J. Coordination studies of 5,6-diphenyl-3-(2-pyridyl)-1,2,4-triazine towards Cu2+ cation. X-ray studies, spectroscopic characterization and DFT calculations. Polyhedron. 2008;27(13):2959–2967. doi: 10.1016/j.poly.2008.05.033. [DOI] [Google Scholar]
- 28.Palivan C. G., Palivan H. M. N., Goodman B. A., Cristescu C. ESR study of some asymmetric-triazine copper(II) complexes having high antiviral activity. Applied Magnetic Resonance. 1998;15(3-4):477–488. doi: 10.1007/bf03162030. [DOI] [Google Scholar]
- 29.Busto N., Valladolid J., Martinez-Alonso M., et al. Anticancer activity and DNA binding of a bifunctional Ru(II) arene aqua-complex with the 2,4-diamino-6-(2-pyridyl)-1,3,5-triazine ligand. Inorganic Chemistry. 2013;52(17):9962–9974. doi: 10.1021/ic401197a. [DOI] [PubMed] [Google Scholar]
- 30.Al-Assy W. H., Mostafa M. M. Comparative studies and modeling structures of two new isomers containing binuclear PdII complexes derived from 2,4,6-tri-(2-pyridyl)-1,3,5-triazine (TPTZ) Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2014;120:568–573. doi: 10.1016/j.saa.2013.10.118. [DOI] [PubMed] [Google Scholar]
- 31.Najafpour M. M., Hołyńska M., Amini M., Kazemi S. H., Lis T., Bagherzadeh M. Two new silver(I) complexes with 2,4,6-tris(2-pyridyl)-1,3,5-triazine (tptz): preparation, characterization, crystal structure and alcohol oxidation activity in the presence of oxone. Polyhedron. 2010;29(14):2837–2843. doi: 10.1016/j.poly.2010.07.005. [DOI] [Google Scholar]
- 32.Marandi F., Jangholi M., Hakimi M., Rudbari H. A., Bruno G. Synthesis and crystal structures of the first cadmium complexes of 3,5,6-tris(2-pyridyl)-1,2,4-triazine ligand. Journal of Molecular Structure. 2013;1036:71–77. doi: 10.1016/j.molstruc.2012.09.070. [DOI] [Google Scholar]
- 33.Mashaly M. M., El-Shafiy H. F., El-Maraghy S. B., Habib H. A. Synthesis, properties and thermal studies of oxorhenium(V) complexes with 3-hydrazino-5,6-diphenyl-1,2,4-triazine, benzimidazolethione and 2-hydrazinobenzimidazole. Mixed ligand complexes, pyrolytical products and biological activity. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2005;61(8):1853–1869. doi: 10.1016/j.saa.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 34.Łakomska I., Golankiewicz B., Wietrzyk J., et al. Synthesis, spectroscopical characterization and the biological activity in vitro of new platinum(II) complexes with imidazo[1,5-a]-1,3,5-triazine derivatives and dimethylsulfoxide. Inorganica Chimica Acta. 2005;358(6):1911–1917. doi: 10.1016/j.ica.2004.12.033. [DOI] [Google Scholar]
- 35.Vzorov A. N., Bhattacharyya D., Marzilli L. G., Compans R. W. Prevention of HIV-1 infection by platinum triazines. Antiviral Research. 2005;65(2):57–67. doi: 10.1016/j.antiviral.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Sun R. W., Ma D. L., Wong E. L., Che C. M. Some uses of transition metal complexes as anti-cancer and anti-HIV agents. Dalton Transactions. 2007;(43):4884–4892. doi: 10.1039/B705079H. [DOI] [PubMed] [Google Scholar]
- 37.Cramer C. J., Truhlar D. G. Density functional theory for transition metals and transition metal chemistry. Physical Chemistry Chemical Physics. 2009;11(46):10757–10816. doi: 10.1039/b907148b. [DOI] [PubMed] [Google Scholar]
- 38.Ranasinghe K., Handunnetti S., Perera I. C., Perera T. Synthesis and characterization of novel rhenium(I) complexes towards potential biological imaging applications. Chemistry Central Journal. 2016;10(1):p. 71. doi: 10.1186/s13065-016-0218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abeydeera N., Perera I. C., Perera T. Synthesis, characterization, and BSA-binding studies of novel sulfonated zinc-triazine complexes. Bioinorganic Chemistry and Applications. 2018;2018:7. doi: 10.1155/2018/7563820.7563820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Institute CaLS. M7–A7. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—seventh edition. [Google Scholar]
- 41.Béreau V., Marrot J. Coordination studies of 5,6-diphenyl-3-(2-pyridyl)-1,2,4-triazine towards Zn2+ cation. Synthesis and characterization by X-ray diffraction and spectroscopic methods. Comptes Rendus Chimie. 2005;8(6-7):1087–1092. doi: 10.1016/j.crci.2004.10.004. [DOI] [Google Scholar]
- 42.Eastwood D., Lidberg R. L., Dresselhaus M. S. Ultraviolet-visible fluorescence spectroscopy of selected polyaromatic hydrocarbons and organometallics on hexagonal graphite and boron nitride. Chemistry of Materials. 1994;6(2):211–215. doi: 10.1021/cm00038a019. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: comparison of UV-Vis data of ferrozine, ferene, and complexes (1)–(4) is presented in a tabulated form in Supplementary Materials.
Data Availability Statement
The data used to support the findings of this study are included within the article and within the Supplentary Information file.