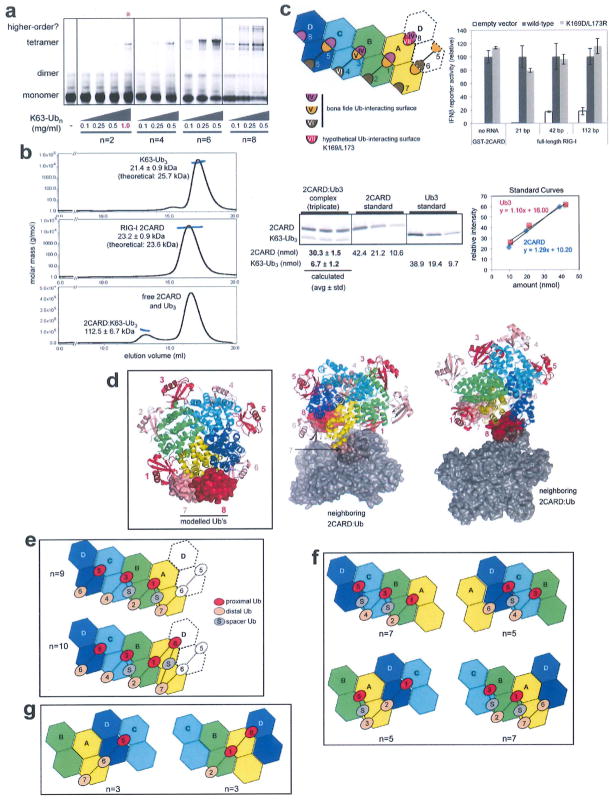
Extended Data Figure 7. High avidity interaction is required for efficient formation of the 2CARD tetramer by K63-Ubn (n > 2).
a, EMSA analysis of the 2CARD tetramer formation using fluorescently labelled 2CARD-S (50 μM) with increasing concentrations of K63-Ubn (n = 2, 4, 6, 8). Note that an additional higher concentration (1 mg ml−1) was included only for K63-Ub2 (red asterisk), due to its low efficiency to stimulate 2CARD tetramerization. With K63-Ub8, additional bands appeared above the tetramer band, possibly reflecting two or more 2CARD tetramers bridged by a single Ub chains. b, Molecular mass analysis of 2CARD in complex with K63-Ub3i using multi-angle light scattering (MALS) coupled to size exclusion chromatography (SEC). Molecular mass estimated for the complex is 112.5 kDa (± 6.7 kDa), which is consistent with a tetrameric 2CARD (92.8 kDa, 23.2 ± 0.9 kDa as a monomer) bound by a single chain of K63-Ub3 (21.4 ± kDa). This 4:1 binding ratio of 2CARD to Ub3 is further supported by the SDS–PAGE intensity analysis of the complex (purified from MALS-SEC above) using Krypton fluorescence stain (right) (mean ± s.d., n = 3), which suggests the molar ratio of 4.5:1 for 2CARD–Ub3. This result suggests the sufficiency of a single chain of K63-Ubn (n > = 3) for stabilizing the 2CARD tetramer, although it does not exclude potential binding of additional Ubs at saturating concentrations3. Note that previous study3 suggesting 4:4 binding of 2CARD to Ubn (n = 3–6) was performed in a buffer lacking salt (20 mM Tris-HCl (pH 7.5) and 1 mM DTT), whereas the current study was performed with 150 mM NaCl (20 mM HEPES (pH 7.5) and 150 mM NaCl), which could be responsible for the divergent results. The sufficiency of the single chain of Ub3 for stabilizing the 2CARD tetramer suggests that multiple stoichiometries and Ubn binding configurations are possible, depending on the concentrations of 2CARD and Ubn as well as buffer compositions. See g for how a single chain of K63-Ub3 could stabilize the 2CARD tetramer. c, Six Ub binding sites (1–6) were occupied in the crystal structure, with a potential for binding of up to eight total Ub molecules in the 2CARD tetramer. Site 7 is equivalent to sites 2, 4 and 6, and thus is a bona fide Ub-binding site. Site 8 is a hypothetical Ub-binding site, as its interaction with Ub would simultaneously utilize surfaces IV and VII, instead of surfaces IV and V as in sites 1, 3 and 5. Mutations of VII (K169D/L173R) did not affect the signalling activity of RIG-I in cells based on the IFNβ reporter assay (right) (mean ± s.d., n = 3). Although this result suggests that surface VII (which only affects site 8, not 1–7) is not important, it does not exclude the possibility of site 8 serving as another Ub-binding site, as the loss of one out of 8 sites may not have a significant effect on the signalling outcome, d, A model of the 2CARD tetramer with all 8 potential Ub binding sites occupied. Ub bound to sites 7 and 8 (surface representation) were modelled by superposing 2CARDs bound to distal and proximal Ub onto 2CARD(A) and (D), respectively. Ub binding sites are numbered according to the 2D representation in c. Crystallographic packing prevents Ub occupancy of sites 7 and 8. Neighbouring molecules, which occlude the sites 7 (left) and 8 (right), are shown in grey surface. e, Two configurations to occupy 7 or 8 potential Ub-binding sites in the 2CARD tetramer using a single K63-Ubn chain. Ub-binding sites are numbered as in b. Ub labelled ‘S’ stands for the unbound Ub that serves as a spacer. The presence of spacer Ub is consistent with the observed activity of Ub4 with K63- and K48- mixed linkage in stimulating RIG-I 2CARD3, as there is no geometric restriction to impose the linkage specificity for the spacer Ub. f, Minimal length of K63-Ubn chain that allows bridging of four 2CARDs by a single Ub chains. Four examples were shown, in which Ub chains start with 2CARD subunit A, B, C or D. Ub-binding sites are numbered as in b. Ub labelled ‘S’ stands for the unbound Ub that serves as a spacer, g, Two configurations that a single chain of K63-Ub3 can bridge three 2CARDs (without involvement of a spacer Ub).