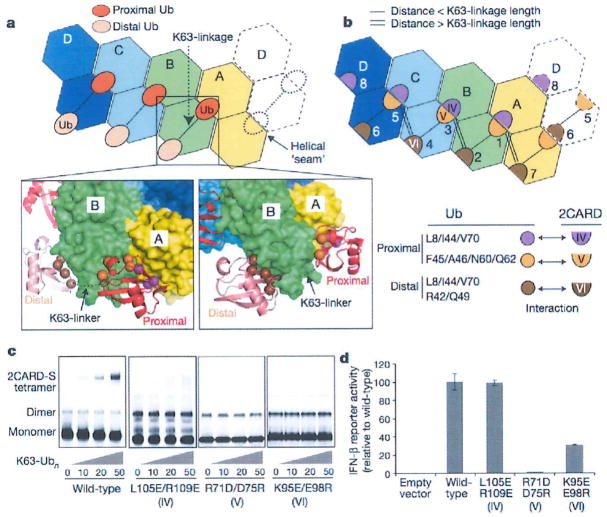
Figure 3. Ub binds to the 2CARD tetramer by decorating the outer rim of the helical trajectory.
a, Two types of Ub–2CARD interactions. Proximal Ub forms a composite interaction with two adjacent 2CARDs using two surface areas (purple and orange spheres). Distal Ub interacts with only one 2CARD using one of the two surface areas (brown spheres), with the exception of Ub bound to D. Dotted lines represent disordered K63-linkers between proximal and distal Ubs. See Extended Data Fig. 6. b, Three types of 2CARD surface areas (IV, V and VI) are involved in Ub binding. Surface IV and VI interact with L8/I44/V70 in proximal and distal Ubs, respectively, whereas surface V interacts with F45/A46/N60/N62 of proximal Ub. Mapping of IV–VI onto the 2D representation of the 2CARD tetramer revealed 7 bona fide Ub binding sites, six of which (sites 1–6) are occupied in our structure, and one potential binding site (site 8, see Extended Data Fig. 7c, d for additional discussion). Single and double lines indicate respectively the distance between adjacent Ubs that can be directly connected through a K63-linkage and those that cannot (thus requiring a spacer Ub). c, EMSA analysis of the tetramer formation by wild-type RIG-I 2CARD-S and its mutants with altered surfaces IV, V and IV, upon incubation with K63-Ubn (n > 8). d, IFN-β reporter activity of wild-type and mutant RIG-I 2CARD in GST fusion (mean ± s.d., n = 3).