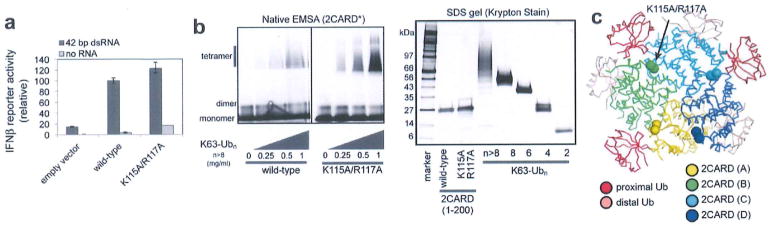
Extended Data Figure 1. RIG-I 2CARD (K115A/R117A) forms the signalling-competent 2CARD tetramer.
a, IFN-β reporter activity of wild-type RIG-I and the K115A/R117A mutant with and without 42 bp dsRNA stimulation (mean ± s.d., n = 3). b, Left, EMSA analysis of tetramerization of wild-type and K115A/R117A RIG-I 2CARD (residues 1–200) with K63-Ubn (n > 8). 2CARD was N-terminally labelled with fluorescein using sortase (see Methods). Right, SDS analysis of wild-type and mutant 2CARD, and K63-Ubn chains used in this study. Unless mentioned otherwise, K63-Ubn indicates the chain length n > 8 throughout the manuscript, c, Mapping of K115 and R117 onto the crystal structure. Although K115 and R117 are located at the edge of the interface, K115A/R117A has little effect on the cellular signalling activity of RIG-I (a) and tetramerization of RIG-I 2CARD (b), indicating that these residues are not critical for mediating inter-domain contacts.