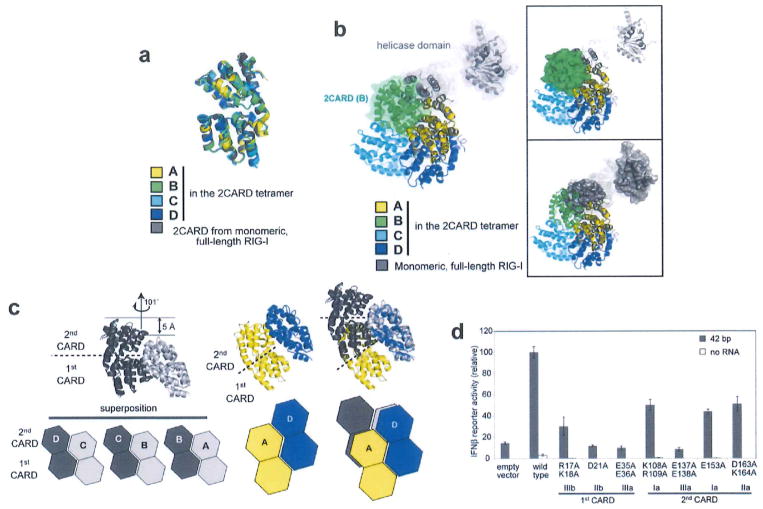
Extended Data Figure 3. Assembly of the 2CARD tetramer is mediated by rigid-body docking along the helical trajectory with the pitch in the screw equivalent to a single CARD.
a, Superposition of RIG-I 2CARD in the tetramer (subunits A–D) and 2CARD from full-length duck RIG-I (PDB: 4A2W). b, Superposition of full-length RIG-I (grey) onto the 2CARD tetramer by aligning 2CARD in full-length RIG-I with 2CARD subunit A (yellow) in the tetramer. The same colour code was used as in a. The superposition shows that the helicase domain in full-length RIG-I masks the 2CARD(A)–2CARD(B) interface and sterically blocks subunit B (green) from interacting with A. On the right, surface representation was separately shown for 2CARD(B) in the tetramer (top) and the helicase domain in full-length RIG-I (bottom) to further demonstrate the steric clash between the helicase domain and 2CARD(B). c, Geometric relationship between adjacent 2CARDs. Superposition of the ‘cut-out’ dimers of A–B, B–C and C–D (left), showing repetition of intermolecular interactions along the helical trajectory. The D–A interaction (middle) in the helical ‘seam’ (as defined in Fig. 1b) differs from the A–B, B–C and C–D interactions by a relative dislocation of A by a single CARD (right), d, IFN-β reporter activity of wild-type RIG-I and tetramer interface mutants (in Fig. 1d) with and without 42 bp dsRNA stimulation in 293T cells (mean ± s.d., n = 3).