Figure 1.
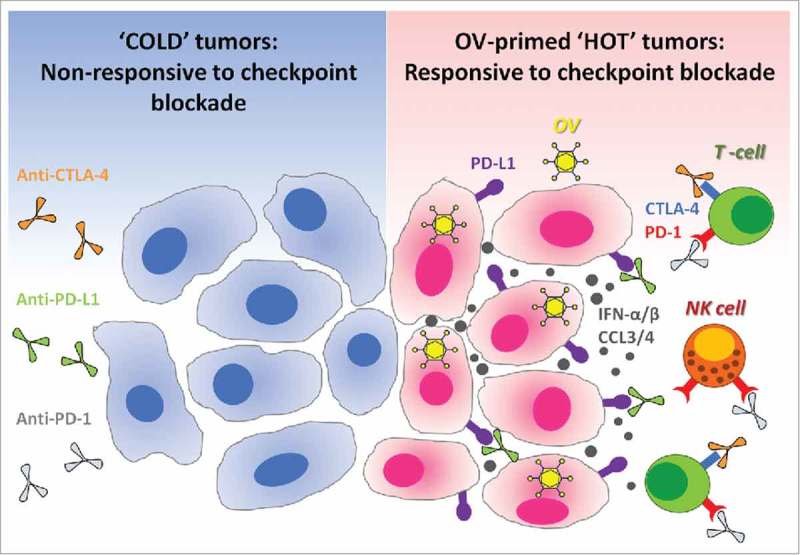
Oncolytic viruses make tumors ‘hot’ and suitable for checkpoint blockade cancer immunotherapies. Immune checkpoint blockade is inefficient in ‘cold’ tumors, which are poorly infiltrated by immune cells and also have low expression of PD-L1 on their surface. In the absence of available targets, immune checkpoint blockers like anti-PD-L1 (targeting PD-L1 expressed at the surface of cancer cells or on antigen-presenting cells), alone or in combination with anti-CTLA-4, remain therapeutically inefficient (left panel). Therapeutic administration of oncolytic viruses (OV) into tumors promotes strong antiviral immune response accompanied by the production of cytokines such as type-1 interferons and chemokines.17,26-28 Type-1 interferons promote the expression of PD-L1 on the surface of cancer cells, while chemokines like CCL3 and CCL4 attract immune cells which often express PD-1 or CTLA-4.29-32 Thus, antiviral immunological events inflame the tumor and make it ‘hot’. When checkpoint inhibitors are administered subsequently, they can bind to their respective targets on either cancer or immune cells. As a final result, oncolytic viruses sensitize tumors to the therapeutic effects of immune checkpoint inhibitors (right panel). OV: Oncolytic virus; NK cell: Natural killer cell; PD-1: Programmed death-1; PD-L1: Programmed death ligand-1; CTLA-4: Cytotoxic T-lymphocyte-associated protein 4; IFN-α/β: Interferon-alpha or beta; CCL3/4: Chemokine (C-C motif) ligand 3 or 4.
