Figure 1.
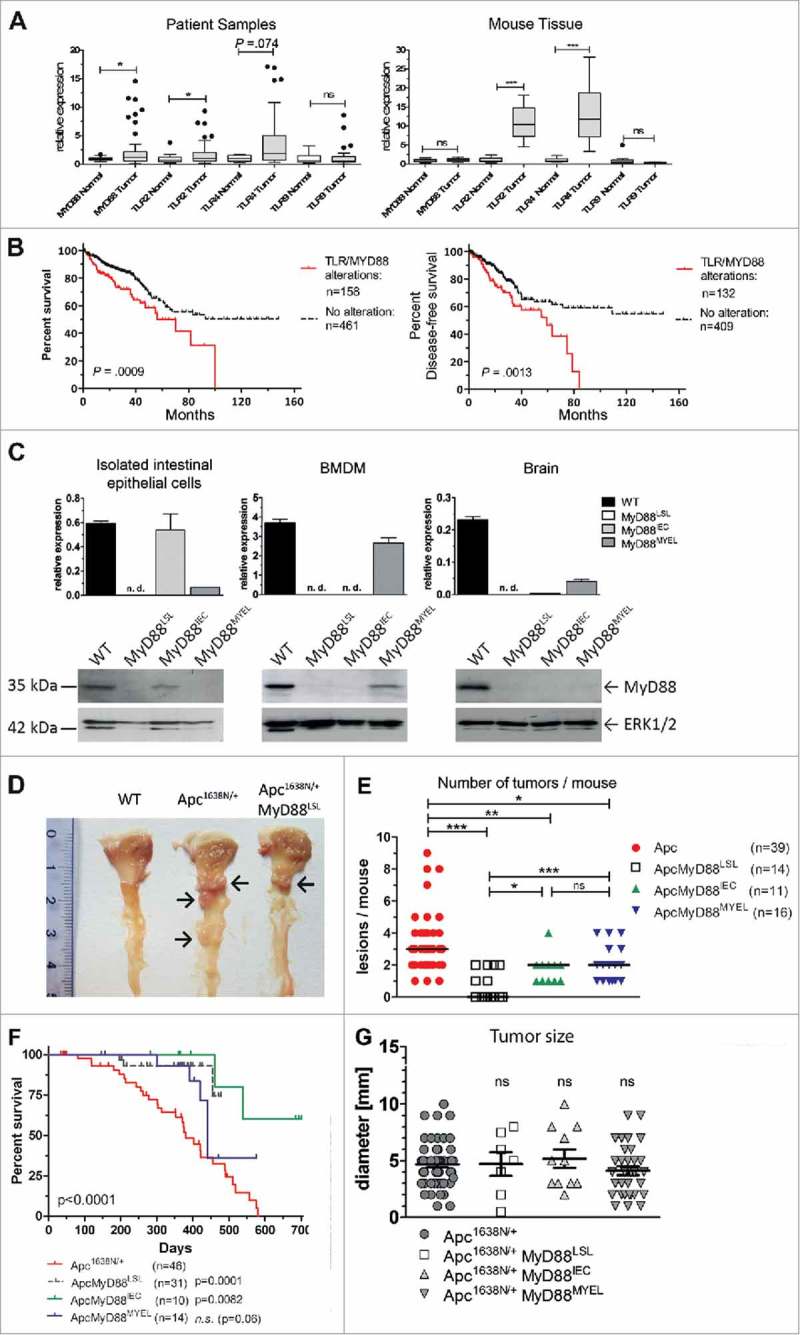
Myd88/TLR signaling is frequently overexpressed in colorectal cancer and associated with prognosis. A, TLR signaling components are upregulated in human (left) and murine (right) intestinal tumors compared to normal mucosa, as verified by qRT-PCR for Myd88, TLR2, TLR4 and TLR9. Human colorectal cancer (n = 51 patients) shows significant upregulation of Myd88 and TLR2 transcripts. Right panel: colon cancer model Apc1638N (n = 15 mice per group) shows a highly significant intratumoral upregulation of TLR2 and TLR4. *P < .05; ***P < .01; ns: not significant. B, Alterations in the TLR pathway are highly significantly associated with poor overall survival (log-rank test: P = .0009), as well as with poor disease-free survival (log-rank test: P = .0013). (C-H) Genetic “switch on” mouse models demonstrate that intestinal carcinogenesis depends on MyD88 expression in both epithelial and myeloid cells. C, Tissue-specific re-expression of Myd88 in intestinal epithelial cells (IEC) was achieved in MyD88IEC mice, or in bone marrow derived macrophages in the MyD88MYEL strain. Expression was analyzed on mRNA level by qRT-PCR (n = 4 mice/group; top panel). No expression was detected in control tissue (brain). Bottom panel: representative example for successful and tissue-specific “switch on” of MyD88 expression on protein level (immunoblot). Loading control: total ERK1/2. D, Macroscopic analysis of representative tissue samples from mice at 12 months of age: wildtype control is tumor-free, Apc1638N/+-model shows several tumors in proximal duodenum (arrows), Apc1638N/+ MyD88LSL mice have strongly reduced tumor formation (arrow). E, Median tumor numbers per animal. Compared to parental line (Apc1638N/+), tumors per animal are significantly reduced in MyD88-deficient mice (Apc1638N/+ MyD88LSL; P = .00018), as well as in mice with re-expression in IECs (Apc1638N/+ MyD88IEC; P = .0123), or in myeloid cells (Apc1638N/+ MyD88LSL; P = .0245). Re-expression of MyD88 in IECs, as well as in myeloid cells is sufficient for a significant, but partial restoration of the tumor phenotype, (Apc1638N/+ MyD88IEC: P = .0256; Apc1638N/+ MyD88MYEL: P = .0037). F, Kaplan-Meier survival analysis show significantly enhanced tumor-specific survival for mice with global MyD88-deficiency, or re-expression of MyD88 in intestinal epithelia, as compared to the parental Apc1638N/+ strain. G, No differences in tumor size were observed. Macroscopically visible lesions were measured along the largest diameter.
