Figure 1.
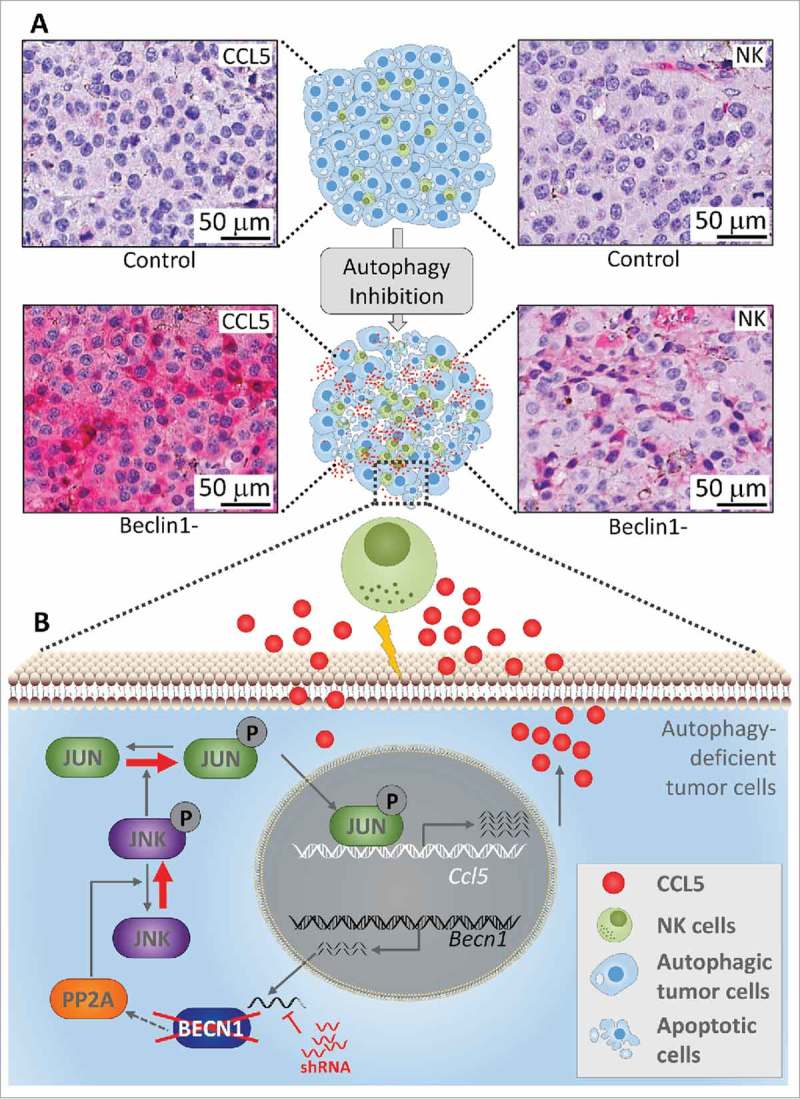
Targeting autophagy induces a massive infiltration of NK cells into melanoma tumors. The upper part (A) shows immunohistochemical staining of NK cells and CCL5 on syngeneic transplanted control or Beclin1-defective B16-F10 melanoma tumor sections. The lower part (B) describes the molecular mechanism underlying the expression of the chemotactic cytokine CCL5. Briefly, shRNA silencing Beclin1 (Becn1) leads to a decrease in the activity of the Protein phosphatase 2A (PP2A) by a mechanism not yet understood. Such a decrease enhances the phosphorylation of JNK which subsequently phosphorylates c-JUN. Phosphorylated c-JUN binds to the promoter of Ccl5 and induces its transcription. CCL5 released by Beclin1-defective tumor cells binds to CCL5 receptor on the surface of NK cells, and induces their infiltration. Functional NK cells recruited to the tumor site kill cancer cells and thereby reduce the tumor volume.
