Figure 3.
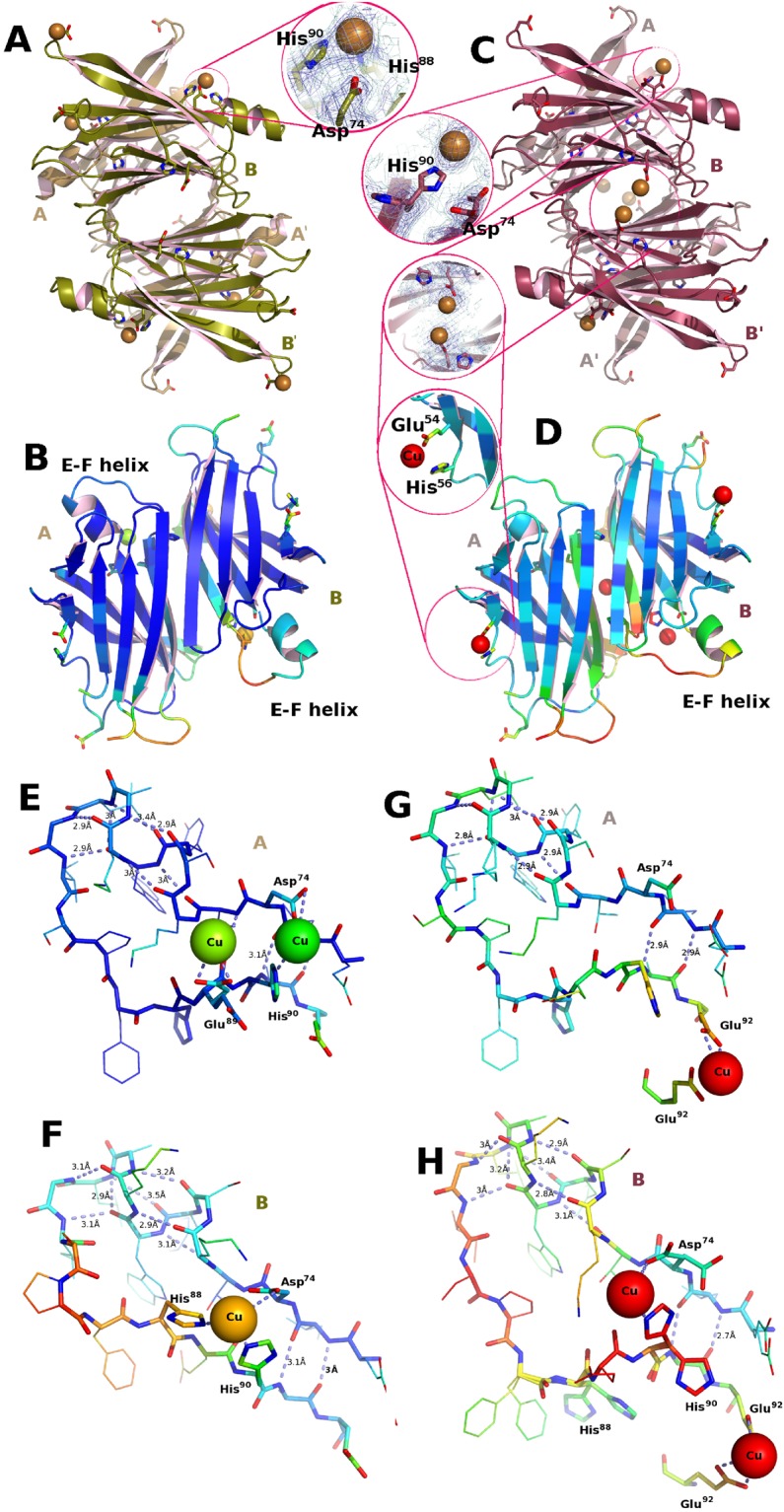
Conformation induced by copper binding to TTR. (A,B) Structure obtained in the absence of Aβ. (C,D) Structure obtained using crystals grown in the presence of Aβ(1–28). Copper soaking into TTR crystals stimulates in both cases a change in conformation similar to that observed for iron and for rhenium. The amplitude of the changes are greater in TTR crystals grown in the absence of Aβ. (A) Overall view of the hetero-conformational A-B/A′-B′ tetramer rebuilt using the 2-fold crystallographic symmetry operation. (B) Crystallographic A-B dimer colored according to B-value to highlight the increased mobility that occurs in the 74–92 stretch that includes the E-F helix. (C) In the crystals obtained in the presence of Aβ(1–28) the positioning of the copper is different from those grown in the absence of the amyloid peptide. The binding is probably weakened from copper chelation by disordered Aβ(1–28) in the solvent channels of the crystal. Residual copper binding is observed in proximity of Asp-74 with minor cooperation of His-90 and at the entrance of the ligand binding channel mediated by Glu-92 with His-56 in proximity. (D) The overall mobility of the protein structure for the TTR crystals grown in the presence of Aβ(1–28) is greater than for those grown without the peptide. (E) In the crystals obtained in the presence of Aβ(1–28) the positioning of the copper is different from those grown in the absence of the amyloid peptide. Residual copper binding is observed in proximity of Asp-74 with minor cooperation of His-90 and at the entrance of the ligand binding channel mediated by Glu-92 with His-56 in proximity. (F) The overall mobility of the structure for the crystals grown in the presence of Aβ(1–28) is greater than for those grown without the peptide. (G) Copper binds only at the entrance of the ligand binding channel on monomer A. (H) The disorder of stretch of residues 72–92 of monomer B is probably due to an incomplete transition towards the conformation observed in (D).
