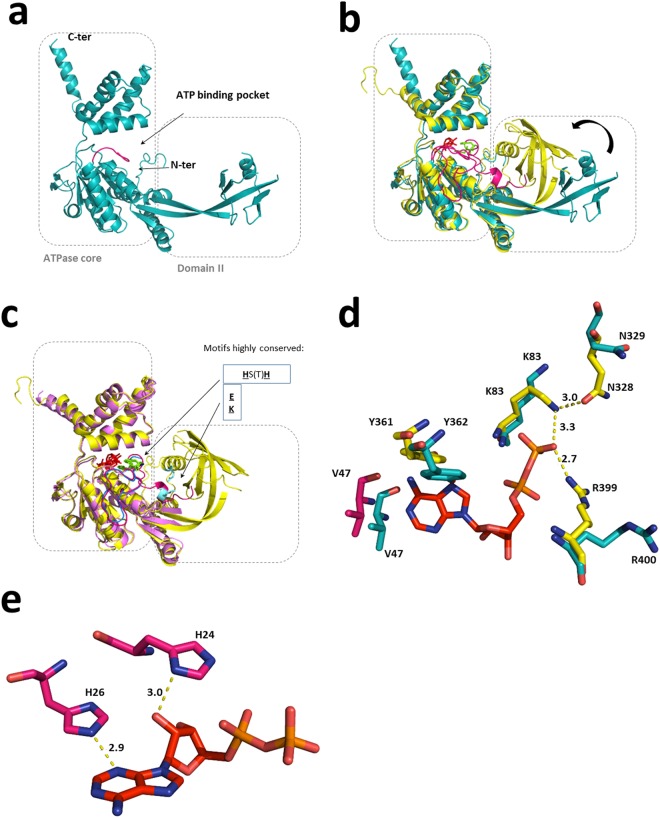
Figure 3.
Comparison between existing structures of RuvBL2 suggests a connection between nucleotide binding and domain II motion. (a) The apo hsRuvBL2 (blue, this work, PDB ID 6H7X) is associated with a stretched, or at least loose conformation of the domain II. (b) The apo RuvBL2 is superimposed with the ADP-bound RuvBL2 from Chaetomium thermophilum (yellow, PDB ID 4WW4). When compared to the apo hsRuvBL2, the ADP-bound ctRuvBL2 displays a positioning of domain II closer to the ATPase core and a more ordered N-terminal loop (pink). ADP is depicted in red sticks. (c) The ATP-bound, domain II-truncated hsRuvBL2 (light pink, PDB ID 2XSZ) is superimposed with the full-length ctRuvBL2 (4WW4). It becomes apparent that the absence of the external part of domain II has an influence in the organization of the N-terminal loop. In both nucleotide-bound forms, the N-terminal loop interacts with the nucleotide through two conserved histidines; however, in the domain II-truncated hsRuvBL2, the N-terminal loop (blue) remains disordered from the first residue up to the place of interaction with the nucleotide. The histidines that interact with the nucleotide are depicted in green and the motif is highlighted; residues involved in electrostatic interactions between the N-terminal loop and domain II are depicted in light blue and highlighted as well. (d) Superimposition of the nucleotide binding pocket of ctRuvBL2 (yellow, 4WW4) with the human RuvBL2 homologous residues (blue, 6H7X – this structure). The ADP molecule (depicted in red) is from the fungal structure. Atoms are coloured as follows: N – dark blue; O – salmon; P – dark yellow. V47 from C. thermophilum is depicted in pink since it is part of the N-terminal loop. (e) Position of conserved histidines 24 and 26 in relation to ADP in the binding pocket of CtRuvBL2. The interatomic distances shown are in Å.