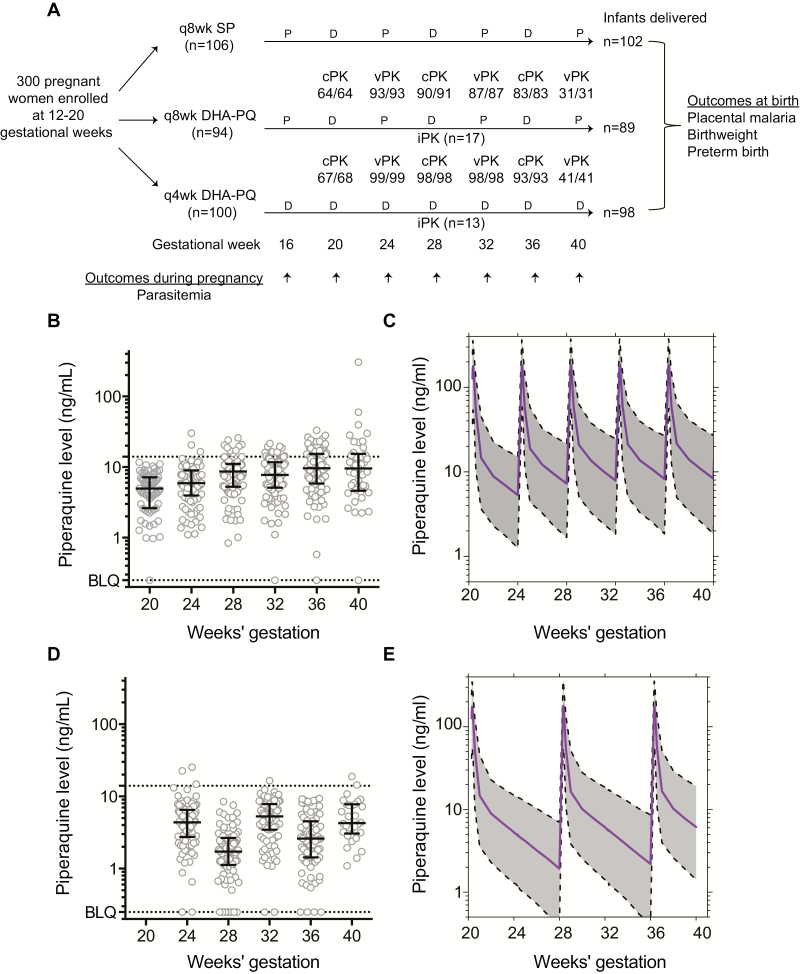
Figure 1.
Trial profile and derivation of population pharmacokinetics (PK) model. A, Trial profile showing women enrolled, randomized, and sampled for piperaquine during pregnancy; infants delivered; and outcomes assessed. Numbers below population PK sampling show samples analyzed/collected at each timepoint. B–E, Raw and modeled PK data between dihydroartemisinin-piperaquine dosing every 4 weeks (B and C) and every 8 weeks (D and E). Abbreviations: BLQ, below the limit of quantitation; cPK, capillary sample; D, active drug; DHA-PQ, dihydroartemisinin-piperaquine; iPK, intensive PK study; P, placebo; q4wk, every 4 weeks; q8wk, every 8 weeks; SP, sulfadoxine-pyrimethamine; vPK, venous sample.