Abstract
A sclerosed hemangioma of the liver is a rare benign lesion characterized by fibrosis and hyalinization of a hepatic cavernous hemangioma as a result of degeneration. This condition has been difficult to correctly diagnose with imaging. Our patient was a 57-year-old man whose computed tomography (CT) scan showed a mass of 45 mm in diameter in the lateral segment. On dynamic contrast-enhanced CT, the lesion was found to comprise peripheral, gradual, and heterogeneous enhanced areas with a central nonenhanced area; malignancy was suspected. On magnetic resonance imaging, the peripheral area showed slight hperintensity on T2-weighted image, and showed a similar intensity on T1- and diffusion-weighted images as compared to the background liver and gradual enhancement, and the presence of abundant fibrous tissue was suspected. Conversely, the central area showed remarkable hyperintensity on T2-weighted images and no enhancement, and degeneration or hyalinization was suspected. The mass showed no uptake of fluorine-18 fludeoxyglucose (FDG). Some imaging findings suspected a benign tumor, and sclerosed hemangioma with abundant fibrosis and hyalinization was pathologically confirmed. Herein, we report a case of sclerosed hemangioma focusing on possible preoperative diagnosis using a combination of multimodality imaging findings—diffusion-weighted imaging and FDG-positron emission tomography imaging.
Keywords: Sclerosed hemangioma, Cavernous hemangioma, Intrahepatic cholangiocarcinoma, Fluorine-18 fludeoxyglucose
1. Introduction
Sclerosed hemangioma of the liver is a rare benign lesion characterized by fibrous replacement and hyalinization of a hepatic hemangioma as a result of degeneration [1], [2], [3], [4]. It has been difficult to correctly diagnose a sclerosed hemangioma with imaging, and in several previous studies, most preoperative diagnoses were intrahepatic cholangiocarcinoma or metastasis [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]. This is because the varying degrees of fibrosis and hyalinization mask the typical signal intensity of magnetic resonance (MR) images and enhanced patterns on the dynamic contrast study, even with the recent advancements in radiological modalities. However, we thought that a combination of multimodality findings, including MR imaging and positron emission tomography (PET), may support the preoperative speculation of a hepatic sclerosed hemangioma. Herein, we report a case of a sclerosed hemangioma with a special focus on possible preoperative diagnoses using a combination of multimodality findings, which are diffusion-weighted images and fluorine-18 fludeoxyglucose (FDG)-PET. In addition, we also discuss the confusion between sclerosed and sclerosing hemangiomas.
2. Case report
A 57-year-old man was referred to our hospital for further examination of a hepatic lesion found during an ultrasound. The patient's family history and past medical history was unremarkable. Laboratory analysis indicated hyperlipidemia (total cholesterol: 249 mg/dL [normal range 145–220 U/mL], triglycerides: 181 mg/dL [normal range 45–150 U/mL]) and others were within normal range. Serum examination for hepatitis B and C infection was negative. Other factors of chronic liver disease, such as alcoholism and nonalcoholic fatty liver disease, were absent. Tumor markers, such as prothrombin induced by the absence of vitamin K or antagonist-II, alpha-fetoprotein, carcinoembryonic antigen, and carbohydrate antigen 19-9, were all negative.
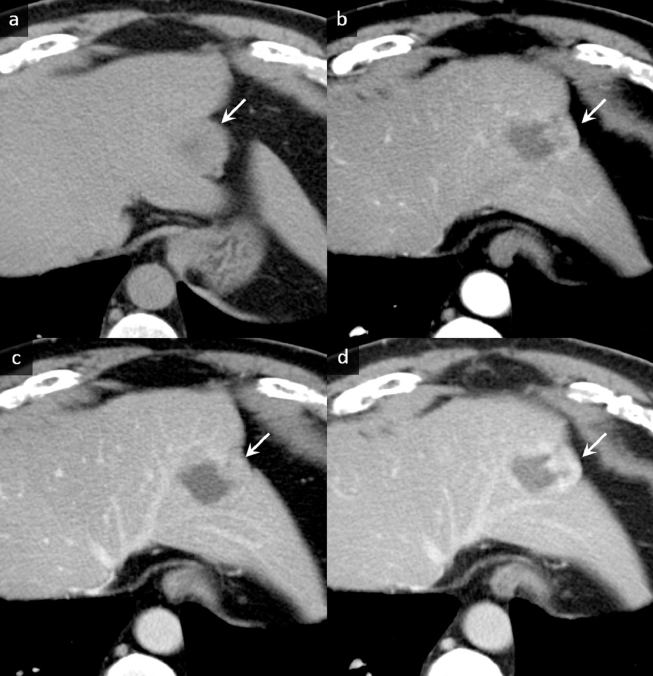
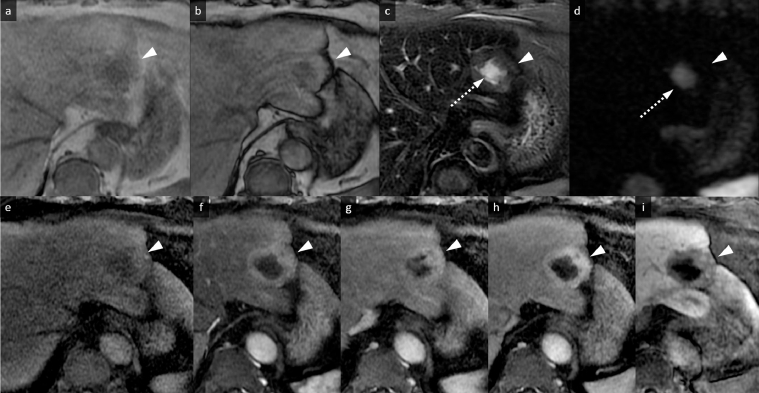
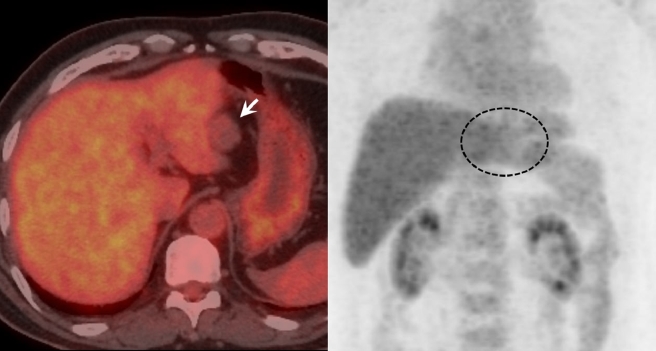
Noncontrast computed tomography (CT) showed diffused, low-density areas in the background liver parenchyma, indicating a mild fatty liver (Fig. 1a). The well-defined, lobulated mass which was 45 mm at its largest diameter and located in lateral segment showed lower density than the background liver (Fig. 1A). On MR imaging, the peripheral area of the mass showed slight hperintensity on fat-suppressed T2-weighted images and showed similar intensity on T1-weighted images as compared to the background liver (Fig. 2a–c). Focal fat deposits within the mass were not identified on T1-weighted images (in and out of phases) (Fig. 2a and b). Diffusion-weighted echo planar images with a high b value of 1000 showed isointensity compared to the background liver (Fig. 2d). Conversely, the central area of the mass showed remarkable hyperintensity on fat-suppressed T2-weighted images and lower intensity on T1-weighted images compared to the background liver and the peripheral area (Fig. 2a–c). Diffusion-weighted images also showed remarkable hyperintensity compared to the peripheral area (Fig. 2d), and this hyperintensity was thought to be a T2 shine-through effect due to the apparent diffusion coefficient (ADC) which was 3.01 × 10−3 mm2/s (not shown). On the dynamic contrast-enhanced CT and MR imaging, the peripheral area showed gradual and inhomogeneous enhancement, whereas the central area was hypovascular and showed no enhancement (Figs. 1b–d and 2e–h). On the hepatobiliary phase of the gadoxetic acid-enhanced MR imaging, the lesion showed hypointensity compared with the background liver, and marginal hyperintensity in the peripheral area was identified, which was thought to be interstitial pooling due to fibrous changes (Fig. 2i). Diffused background fat deposits in the liver were identified on T1-weighted images in and out of phases, as seen in Fig. 2a and b. The retraction of the liver parenchyma adjacent to the mass was identified (Figs. 1 and 2). No uptake of FDG was identified in the mass on PET-CT (Fig. 3).
Fig. 1.
Computed tomography (CT) images. (a) Noncontrast CT shows diffuse low-density areas in the background liver parenchyma, indicating a mildly fatty liver. The well-defined, lobulated mass, which is 45 mm at its largest diameter and is located in the lateral segment, shows isodensity compared to the background liver parenchyma (arrow) accompanied with a central hypodense area. (b–d) On dynamic contrast-enhanced CT images, gradual enhancement is observed in the peripheral area (b. arterial phase image, c. portal phase image, d. delayed phase images) as shown by arrows. The traction of the liver parenchyma adjacent to the mass is identified.
Fig. 2.
Magnetic resonance (MR) images. (a and b) T1-weighted images (in and out of phases) (a. in phase, b. out of phase). The peripheral area of the mass is isointense accompanied with a central hypointense area (arrowheads), and focal fat deposits within the mass are not identified. (c) Fat-suppressed T2-weighted image. The peripheral area of the mass is slightly hyperintense (arrowhead), accompanied by a central remarkable hyperintense area (arrow) compared to the background liver. (d) Diffusion-weighted image. The peripheral area of the mass is isointense compared to the background liver (arrowhead). The central area of the mass shows remarkable hyperintensity (arrow) which is the T2 shine-through effect. (e) Precontrast T1-weighted image with fat suppression. The mass shows peripheral isointensity and central hypointensity (arrowhead). (f–h) Dynamic contrast-enhanced study. The peripheral area shows gradual and inhomogeneous enhancement, whereas the central area shows hypovascular and no enhancement (arrowheads). (i) Hepatobiliary phase image. The lesion demonstrates hypointensity compared to the background liver (arrowhead).
Fig. 3.
Positron emission tomography-computed tomography (PET-CT) image. No uptake of fluorine-18 fludeoxyglucose (FDG) is identified in the mass on the PET-CT image.
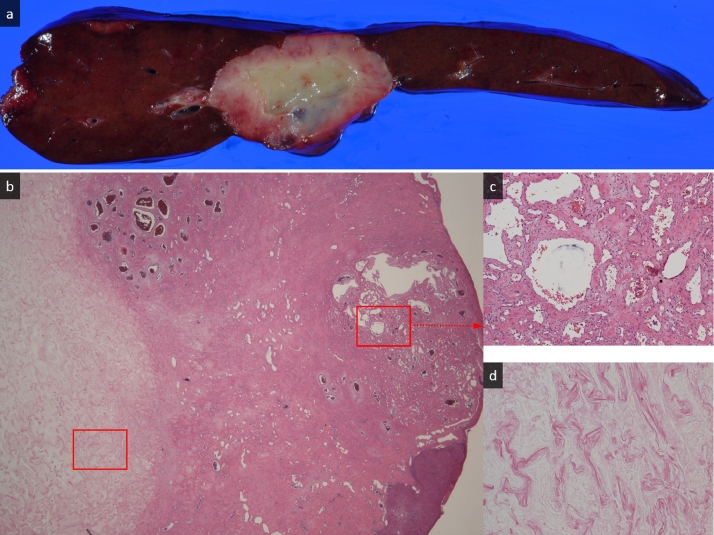
Radiologically, the CT images indicated a malignant tumor, particularly a intrahepatic cholangiocarcinoma. MR images were almost compatible with the tumor accompanied with abundant fibrous changes and degeneration; however, fat-suppressed T2- and diffusion-weighted images were slightly different from the typical intrahepatic cholangiocarcinoma. No uptake of FDG definitively indicated a benign tumor with degeneration or hyalinization, such as a sclerosed hemangioma. However, the possibility of malignancy including liver metastasis or intrahepatic cholangiocarcinoma could not be excluded, and the laparoscopic lateral lobe resection was performed after obtaining a full informed consent. The resection specimen showed a solid mass which had a peripheral red–brown area including a central white area with a smooth margin without a capsule measuring 4.8 × 3.6 × 2.8 cm (Fig. 4a). Histopathologically, the mass mainly comprised collagen fibers with varying sizes of scant endothelial cell-lined vascular spaces, whereas the central area mainly comprised hyalinized tissue on the hematoxylin eosin section (Fig. 4b–d). There was no cell with atypia or nuclear abnormalities. From these characteristics, the mass was diagnosed as a hepatic sclerosed hemangioma.
Fig. 4.
Macroscopic and microscopic findings of the resected tumor. (a) The resection specimen shows a solid mass measuring 4.8 × 3.6 × 2.8 cm with a peripheral red–brown area including a central white area with a smooth margin without capsule. (b) Histopathologically, the tumor comprises fibrous connective tissue highlighted with collagen fibers and various sizes of cavernous hemangioma tissue (right side of the image). The central area of the mass comprises completed hyaline degeneration (hematoxylin eosin [HE], original magnification, ×20) seen on the left side of the image. (c) Various sized vascular spaces with endothelial cell lining and some components of connective tissues are seen in the peripheral area of the mass (HE, original magnification, ×100). (d) The central area of the mass comprises completed hyaline degeneration (HE, original magnification, ×100).
3. Discussion
Here, we report the case of a middle-aged man with a hepatic sclerosed hemangioma, which is a rare type of hepatic hemangioma [1], [2], [3], [4]. Cavernous hemangioma of the liver is the most frequent benign hepatic tumor and usually contains various sizes of vascular channels lined by a single layer of endothelial cells supported by a collagenous wall [15], [16]. This distinctive structure shows a high characteristic signal intensity on T2-weighted images and a hemodynamic characteristic pattern on enhanced CT or MR imaging, and imaging modalities are highly reliable for diagnosis [17], [18], [19], [20]. However, there are some cases that contain parts of hyaline degeneration, which occurs secondary to thrombus, necrosis, or cicatrization [1], [2], [3], [4], and is called “sclerosed hemangioma.” This may result in various uncharacteristic findings on radiological imaging [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]. CT and MR imaging features that are suggestive of sclerosed hemangioma include a geographic pattern, capsular retraction, reduction in size over time, and loss of contrast enhancement, and additional characteristic features include the presence of transient hepatic attenuation difference, ring enhancement, and nodular regions of intense enhancement, as seen in a typical hemangioma [4]. However the imaging diagnosis has still been difficult to differentiate from malignant tumors, including intrahepatic cholangiocarcinoma and liver metastasis, because a sclerosed hemangioma could show variable MR imaging appearance with various degrees of degeneration, fibrosis, and hyalinization [17]. In fact, all previous cases were preoperatively misdiagnosed as malignant tumors [5], [6], [7], [8], [9], [10], [11], [12], [13], [14].
Some earlier reports have demonstrated the feasibility of using diffusion-weighted imaging for the detection and characterization of liver tumors [21], [22]. In particular, diffusion-weighted image-based measurement of an ADC value can contribute to differentiating between benign and malignant lesions of the liver [21], [22]. Furthermore, Hida et al. [7] reported that the diagnostic value of diffusion-weighted imaging for a sclerosed hemangioma and a high ADC value due to the presence of many large vascular spaces in the tumor, therefore ADC value could discriminate sclerosed hemangioma from intrahepatic cholangiocarcinoma and metastasis. In our case, the peripheral area with gradual enhancement showed an intensity similar to that of the background liver on diffusion-weighted imaging, and this finding indicated a lower possibility of a malignant tumor based on the previous report [7], [21], [22], and the hyperintensity of the central area reflected the hyalinization and it was T2 shine-through. However, the possibility of a malignancy could not be excluded considering the echo planar imaging artifacts including ghosting and distortion.
The radiological features revealed by dynamic CT and MR imaging resembled those of hepatic malignancies, leading to a preoperative misdiagnosis. Conversely, FDG-PET could have detected the possible benign character of this sclerosed hemangioma. As with our case, several earlier reports showed no accumulation of FDG [5], [11], [12], [13], [23], [24], [25], [26], [27]. Theoretically, a hemangioma and a sclerosed hemangioma would not increase glucose metabolism. FDG-PET could be helpful in preoperative diagnosis to distinguish a benign sclerosed hemangioma from malignant tumors. Furthermore, according to the findings of MR images, particularly diffusion-weighted images, we strongly suspected a sclerosed hemangioma rather than a malignant tumor.
Percutaneous biopsy of the lesion can be suggested if definitive diagnosis of the lesion cannot be provided by multimodality imaging as an alternative, prior to surgery to distinguish the sclerosed hemangioma from metastasis and intrahepatic cholangiocarcinoma. In the present case, surgical operation was selected because of relative low risk of resection of the lateral segment and the risk of sampling error of biopsy.
In conclusion, we report a rare hepatic benign tumor, which is a hepatic sclerosed hemangioma. Although preoperative diagnosis of sclerosed hemangioma has been difficult on radiological imaging, a combination of multimodalities may allow for a correct preoperative diagnosis. Further case accumulation is required for elucidation of the utility of diffusion-weighted and FDG-PET imaging findings.
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest in association with this study.
Human rights
All of the procedures were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent for publication was obtained from the patient.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2018.04.007.
Appendix. Supplementary materials
References
- 1.Aibe H, Honda H, Kuroiwa T. Sclerosed hemangioma of the liver. Abdomen Imaging. 2001;26:496–499. doi: 10.1007/s002610000202. [DOI] [PubMed] [Google Scholar]
- 2.Cheng HC, Tsai SH, Chiang JH, Chang CY. Hyalinized liver hemangioma mimicking malignant tumor at MR imaging. Am J Roentgenol. 1995;165:1016–1017. doi: 10.2214/ajr.165.4.7676959. [DOI] [PubMed] [Google Scholar]
- 3.Ishii T, Takahara O, Sano I. Sclerosing hemangioma of the liver. Nagasaki Med J. 1995;70:23–26. [Google Scholar]
- 4.Doyle DJ, Khalili K, Guindi M, Atri M. Imaging features of sclerosed hemangioma. Am J Roentgenol. 2007;189:67–72. doi: 10.2214/AJR.06.1076. [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto S, Oshita A, Daimaru Y, Sasaki M, Ohdan H, Nakamitsu A. Hepatic sclerosed hemangioma: a case report and review of the literature. BMC Surg. 2015;17(15)):45. doi: 10.1186/s12893-015-0029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimada Y, Takahashi Y, Iguchi H. A hepatic sclerosed hemangioma with significant morphological change over a period of 10 years: a case report. J Med Case Rep. 2013;28(7)):139. doi: 10.1186/1752-1947-7-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hida T, Nishie A, Tajima T, Taketomi A, Aishima S, Honda H. Sclerosed hemangioma of the liver: possible diagnostic value of diffusion-weighted magnetic resonance imaging. Jpn J Radiol. 2010;28:235–238. doi: 10.1007/s11604-009-0407-3. [DOI] [PubMed] [Google Scholar]
- 8.Park SM, Shin SM, Seo HE. A case of sclerosed hemangioma mimicking intrahepatic cholangiocarcinoma. Korean J Gastroenterol. 2009;54:399–403. doi: 10.4166/kjg.2009.54.6.399. [DOI] [PubMed] [Google Scholar]
- 9.Mori H, Ikegami T, Imura S. Sclerosed hemangioma of the liver: report of a case and review of the literature. Hepatol Res. 2008;38:529–533. doi: 10.1111/j.1872-034X.2007.00306.x. [DOI] [PubMed] [Google Scholar]
- 10.Wakasugi M, Ueshima S, Tei M. Multiple hepatic sclerosing hemangioma mimicking metastatic liver tumor successfully treated by laparoscopic surgery: report of a case. Int J Surg Case Rep. 2015;8C:137–140. doi: 10.1016/j.ijscr.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song JS, Kim YN, Moon WS. A sclerosing hemangioma of the liver. Clin Mol Hepatol. 2013;19:426–430. doi: 10.3350/cmh.2013.19.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin YM. Sclerosing hemangioma in the liver. Korean J Hepatol. 2011;17:242–246. doi: 10.3350/kjhep.2011.17.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin SY. Sclerosed hemangioma of the liver. Korean J Hepatol. 2010;16:410–413. doi: 10.3350/kjhep.2010.16.4.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamashita Y, Shimada M, Taguchi K. Hepatic sclerosing hemangioma mimicking a metastatic liver tumor: report of a case. Surg Today. 2000;30:849–852. doi: 10.1007/s005950070072. [DOI] [PubMed] [Google Scholar]
- 15.Prabhu VR, Burt AR. Pathology of liver tumors. Surgery (Oxford) 2007;25:10–15. [Google Scholar]
- 16.Goodman ZD. Benign tumors of the liver. In: Okuda K, Ishak KG, editors. Neoplasms of the liver. Springer-Verlag; Tokyo: 1987. pp. 105–125. [Google Scholar]
- 17.Ridge CA, Shia J, Gerst SR, Do RK. Sclerosed hemangioma of the liver: concordance of MRI features with histologic characteristics. J Magn Reson Imaging. 2014;39:812–818. doi: 10.1002/jmri.24228. [DOI] [PubMed] [Google Scholar]
- 18.Jang HJ, Kim TK, Lim HK. Hepatic hemangioma: atypical appearances on CT, MR imaging, and sonography. Am J Roentgenol. 2003;180:135–141. doi: 10.2214/ajr.180.1.1800135. [DOI] [PubMed] [Google Scholar]
- 19.Caseiro-Alves F, Brito J, Araujo AE. Liver hemangioma: common and uncommon findings and how to improve the differential diagnosis. Eur Radiol. 2007;17:1544–1554. doi: 10.1007/s00330-006-0503-z. [DOI] [PubMed] [Google Scholar]
- 20.Vilgrain V, Boulos L, Vullierme MP. Imaging of atypical hemangiomas of the liver with pathologic correlation. RadioGraphics. 2000;20:379–397. doi: 10.1148/radiographics.20.2.g00mc01379. [DOI] [PubMed] [Google Scholar]
- 21.Bruegel M, Holzapfel K, Gaa J. Characterization of focal liver lesions by ADC measurements using a respiratory triggered diffusion-weighted single-shot echo-planar MR imaging technique. Eur Radiol. 2008;18:477–485. doi: 10.1007/s00330-007-0785-9. [DOI] [PubMed] [Google Scholar]
- 22.Namimoto T, Yamashita Y, Sumi S, Tang Y, Takahashi M. Focal liver masses: characterization with diffusion-weighted echo-planar MR imaging. Radiology. 1997;204:739–744. doi: 10.1148/radiology.204.3.9280252. [DOI] [PubMed] [Google Scholar]
- 23.Morikawa M, Ishida M, Iida A, Katayama K, Yamaguchi A, Imamura Y. Sclerosed hemangioma of the liver mimicking metastatic liver cancer. J Jpn Surg Assoc. 2005;66:1698–1702. [Google Scholar]
- 24.Iida H, Tango Y, Tsutamoto Y. A resected case of Sclerosed hemangioma. Jpn J Gastroenterol Surg. 2006;39:1493–1497. [Google Scholar]
- 25.Yoshida T, Sugimachi K, Gion T. A case of sclerosing hemangioma mimicking a malignant liver tumor. J Clin Surg. 2010;65:451–455. [Google Scholar]
- 26.Mikami J, Tominaga M, Sendo H. A case of hepatic sclerosing hemangioma mimicking a malignant liver tumor. J Jpn Surg Assoc. 2001;72:965–971. [Google Scholar]
- 27.Yamada S, Shimada M, Utsunomiya T. Hepatic sclerosed hemangioma which was misdiagnosed as metastasis of gastric cancer: report of a case. J Med Invest. 2012;59:270–274. doi: 10.2152/jmi.59.270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.