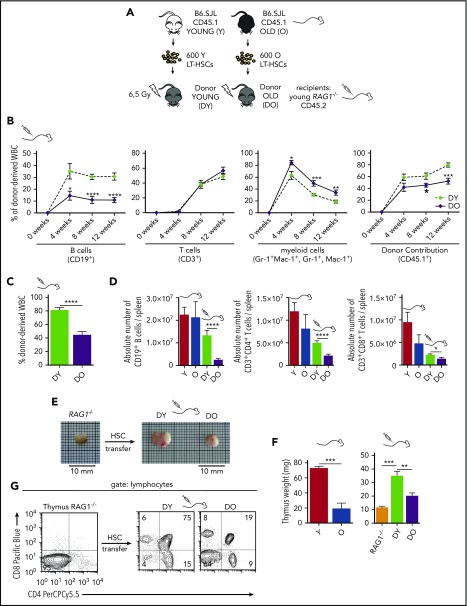
Figure 1.
Transplantation of DY and DO HSCs in RAG1−/−recipients. (A) Schematic representation of the experimental setup: 600 HSCs were isolated from young (Y) and old (O) B6.SJL (CD45.1) mice and subsequently transplanted into irradiated young RAG1−/− (CD45.2) recipients. (B) Contribution of DY and DO HSCs (CD45.1) to white blood cells was analyzed in peripheral blood every 4 weeks up to 12 weeks (DY, DO, n = 15). (C) 12 weeks posttransplantation, recipient mice were scarified and donor contribution to total white blood cells in spleen was analyzed (DY, n = 16; DO, n = 19). (D) Absolute number of donor-derived splenic CD19+ B cells, CD3+CD4+ and CD3+CD8+ T cells in recipient animals and nontransplanted young and aged B6.SJL mice (Y, n = 8; O, n = 7; DY, n = 16; DO, n = 19). (E) Representative gross anatomy of thymi taken from a nontransplanted RAG1−/− mouse and RAG1−/− recipients transplanted with DY or DO HSCs. (F) Weight of thymus glands isolated from nontransplanted young and aged B6.SJL mice, RAG1−/− mice, and RAG1−/− recipients transplanted with DY and DO HSCs (Y, n = 4; O, RAG1−/−, n = 3; DY, n = 5; DO, n = 8). (G) Representative thymic profile of nontransplanted RAG1−/− mice and RAG1−/− hosts of DY and DO HSCs. Total thymic cells from transplanted and nontransplanted animals were analyzed for CD4 and CD8 expression, using flow cytometry. CD4+, CD8+, and CD4+CD8+ T cells were gated as mononucleated cells within the lymphocyte population. *P < .05; **P < .01; ***P < .001; and ****P < .0001. (B) Two-tailed unpaired Student's t-test, mean ± SEM. (C-D) Two-tailed unpaired Student's t-test, mean + SEM. (D) Only means of nontransplanted young vs aged mice and recipients of DY vs DO HSCs were compared statistically. (F) Two-tailed unpaired Students's t-test, one-way ANOVA, mean + SEM.