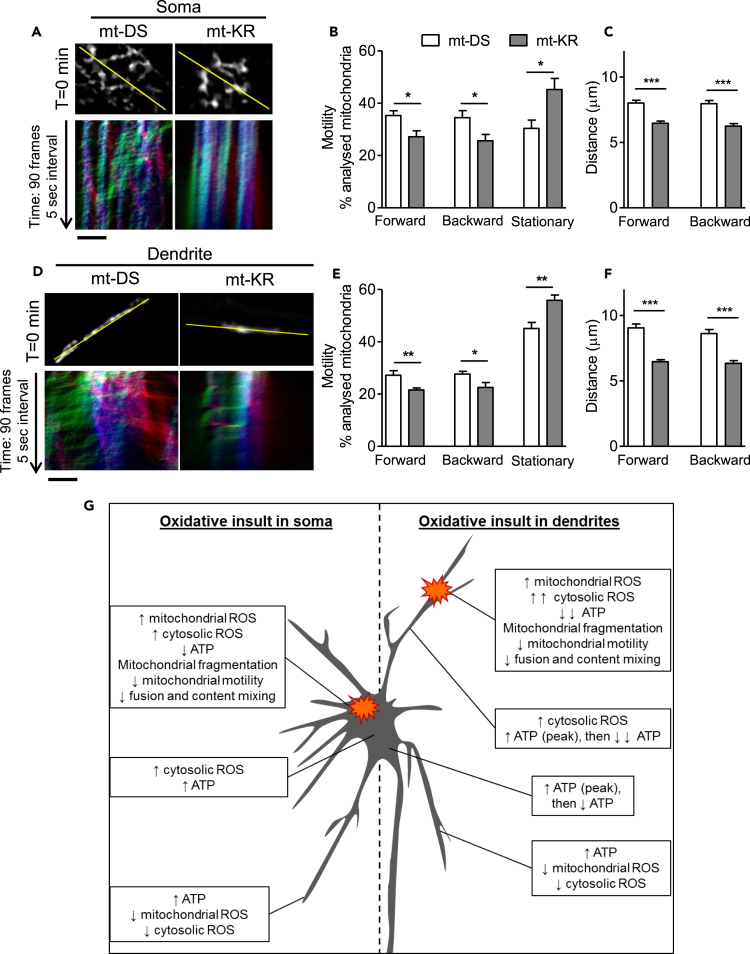
Figure 7.
Decreased Mitochondrial Motility at the Site of Insult after Focal mt-KR Activation, and Proposed Model
(A) Top panel: mt-paGFP fluorescence signal in the soma in the first frame of the time-lapse recording (T = 0 min, total 90 frames, 0.5-s interval). The yellow lines demarcate the selections used to generate kymographs. Lower panel: Representative kymographs (mt-paGFP fluorescence signal) showing the relative mitochondrial motility in the soma after mt-paGFP activation. The blue vertical lines represent stationary mitochondria, the green oblique lines indicate mitochondria moving forward, and the red oblique lines indicate mitochondria moving backward. Scale bar represents 5 μm.
(B) Analysis of mitochondrial motility by direction of movement (forward, backward, and stationary) in the soma. Values represent the mean ± SEM of the proportion of mobile and immobile mitochondria per total number of analyzed mitochondria per kymograph. N = 10–11 cells/group from three independent hippocampal cultures.
(C) Analysis of the distance traveled by mobile mitochondria in the soma. On average 150–200 mitochondria were analyzed per group (n = 10–11 neurons/group from 3 independent hippocampal cultures).
(D) Top panel: mt-paGFP fluorescence signal in the dendrite in the first frame of the time-lapse recording (T = 0 min, total 90 frames, 0.5-s interval). The yellow lines show the selections used to generate kymographs. Lower panel: Representative kymographs (mt-paGFP fluorescence signal) showing relative mitochondrial motility in the dendrite after mt-paGFP activation. Blue vertical lines represent stationary mitochondria, the green oblique lines indicate mitochondria moving forward (toward distal dendrites), and the red oblique lines indicate mitochondria moving backward (back to the soma). Scale bar represents 5 μm.
(E) Analysis of mitochondrial motility by direction of movement (forward, backward, and stationary) in the dendrite. Values represent the mean ± SEM of the proportion of mobile and immobile mitochondria per total number of analyzed mitochondria per kymograph. N = 10–11 cells/group from 3 independent hippocampal cultures.
(F) Analysis of the distance traveled by mobile mitochondria in the dendrite. On average 150–200 mitochondria were analyzed per group (n = 10–11 neurons/group from 3 independent hippocampal cultures).
(B, C, E, and F) Values represent the mean ± SEM Student's paired t test, *p < 0.05; **p < 0.01; ***p < 0.001.
(G) Proposed model of the somatodendritic regulation of redox state and ATP turnover after an acute local oxidative insults. When an oxidative damage occurs within somatic mitochondria (left panel), ROS increase only in the photostimulated mitochondria and the ATP/ADP ratio decreases. Cytosolic ROS levels increase in the damaged region as well as in the remainder of the soma. Impaired mitochondria are fragmented and have a decreased motility and a reduced ability to fuse with other mitochondria. In the soma and dendrites, the ATP/ADP ratio increases, whereas mitochondrial and cytosolic ROS decrease in the distal dendrites. Similarly, when oxidative damage occurs within dendritic mitochondria (right panel), ROS increase only in the photostimulated mitochondria. The ATP/ADP ratio decreases and cytosolic ROS increase in the damaged region to a greater extent than in the soma under similar conditions. Again, impaired mitochondria are fragmented and have a decreased motility and a reduced ability to fuse with other mitochondria. In the rest of the affected dendrite, the ATP/ADP ratio increases and then strongly decreases over time. In the non-damaged dendrites, mitochondrial and cytosolic ROS decrease, with a concomitant increase in the ATP/ADP ratio. Thus, neurons avoid the spread of oxidation by quarantining damaged mitochondria at the site of insult.