Table 1.
Optimization of Conditions for Visible-Light Photoredox Olefination
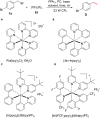 | |||||
|---|---|---|---|---|---|
| Entry | PC | Base (equiv) | Solvent | Time (h) | Yielda |
| 1 | A | Cs2CO3 | CH3CN | 6 | 93 |
| 2 | B | Cs2CO3 | CH3CN | 6 | 21 |
| 3 | C | Cs2CO3 | CH3CN | 6 | 89 |
| 4 | D | Cs2CO3 | CH3CN | 6 | 67 |
| 5 | A | K2CO3 | CH3CN | 6 | 93 |
| 6 | A | NA2CO3 | CH3CN | 6 | 61 |
| 7b | A | – | CH3CN | 6 | 11 |
| 8c | A | K2CO3 | CH3CN | 6 | 92 |
| 9d | A | K2CO3 | CH3CN | 6 | 80 |
| 10e | A | K2CO3 | CH3CN | 6 | NR |
| 11 | A | K2CO3 | CH3CN | 4 | 93 |
| 12 | A | K2CO3 | CH3CN | 3 | 88 |
| 13 | A | K2CO3 | DMF | 4 | 90 |
| 14 | A | K2CO3 | DMA | 4 | 74 |
| 15 | A | K2CO3 | DMSO | 4 | 43 |
| 16 | A | K2CO3 | CH2Cl2 | 4 | 45 |
| 17f | A | K2CO3 | CH3CN | 4 | 84 |
| 18g | A | K2CO3 | CH3CN | 9 | 90 |
| 19h | A | K2CO3 | CH3CN | 4 | Trace |
| 20i | – | K2CO3 | CH3CN | 4 | Trace |
| 21j | A | K2CO3 | CH3CN | 4 | Trace |
Reaction conditions: Ar atmosphere and irradiation of visible light with 23-W CFL, 4-bromobenzyl bromide (1a) (1.0 mmol), paraformaldehyde (2a) (2.0 mmol, relative to amount of formaldehyde), triphenylphosphine (PPh3) (1.5 mmol), photocatalyst (5.0 μmol), base (1.5 mmol), solvent (10 mL), temperature (room temperature ∼25 oC), time 3–6 hr, in a sealed Schlenk tube.
PC, photocatalyst; CFL, compact fluorescent light; DMA, N,N-dimethylacetamide; NR, no reaction.
Isolated yield.
No base.
In the presence of 2 equiv of PPh3.
In the presence of 1 equiv of PPh3.
No PPh3.
Using aqueous formaldehyde (2b) (37% aqueous solution) (2.0 mmol) instead of paraformaldehyde (2a).
Under irradiation of 5-W blue LED light for 9 hr.
The reaction was carried out in air.
No photocatalyst.
No light.
