Primary effusion lymphoma (PEL) is a rare non-Hodgkin lymphoma (NHL) associated with human herpesvirus 8 (HHV8) and increased incidence among HIV-positive individuals. PEL rarely presents as solid tumor masses, or extracavitary PEL (EPEL)1. We found one case of PEL and no EPEL reported from sub-Saharan Africa (SSA)2, despite high prevalence of HIV and HHV8. With improved lymphoma diagnostic infrastructure in Malawi, we recently diagnosed the first two cases of EPEL, out of 85 HIV-associated aggressive NHL cases during 2013–2017.
Case 1 was a 33-year-old who began antiretroviral therapy (ART) nine months before EPEL diagnosis. CD4 count at EPEL diagnosis was 131 cells/μL and HIV RNA was not detectable. The patient had six months of bulky cervical lymphadenopathy, and no B symptoms. Case 2 was a 45-year-old who began ART 11 years prior to EPEL diagnosis. CD4 count at EPEL diagnosis was 220 cells/μL and HIV RNA was 63 copies/mL. The patient had six months of bulky diffuse lymphadenopathy, with B symptoms. Neither patient had other evident disease manifestations of HHV8.
Biopsies were characterized using hematoxylin and eosin (H&E) staining and immunohistochemistry (IHC), and reviewed during weekly telepathology conference with United States hematopathologists. Both neoplasms exhibited a nest-like growth pattern of large pleomorphic cells with prominent nucleoli and frequent mitotic figures, raising the possibility of a solid tumor [Figure 1A]. Case 2 showed loosely arranged cells with a more alveolar pattern and plasmacytoid appearance [Figure 1B]. Both cases were positive by IHC for latency-associated nuclear antigen (LANA) [Figure 1C], MUM-1, MYC (>90%) with high Ki67 >95% [Figure 1D] but negative for CD45, CD20, CD3, CD30, BCL-2, BCL-6, and PAX-5. CD138 was positive in Case 1 but negative in Case 2. Both cases were positive for Epstein-Barr virus (EBV)-encoded RNA (EBER) by in situ hybridization (ISH). Case 1 had high EBV (5.44 log10copies/mL) and KSHV (4.14 log10copies/mL) plasma loads at baseline; these data were not available for Case 2.
Figure 1.
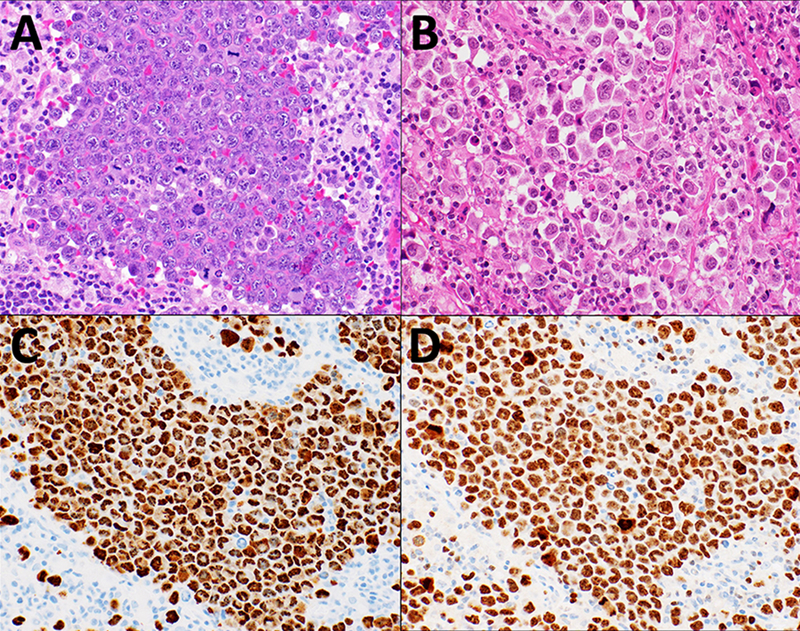
Cervical lymph node biopsy microphotographs from Case 1 and Case 2, A. Case 1 showing tumor cells with nest-like growth pattern of large pleomorphic cells, with prominent nucleoli and frequent mitotic figures (H&E, 40X) B. Case 2 showing more loosely arranged cells with an alveolar pattern and plasmacytoid appearance (H&E, 40X). C. Case 1 with HHV8 LANA IHC staining of the tumor cells showing characteristic punctuate nuclear staining pattern (40X). D. Case 1 with tumor cells showing with very high Ki67% (40X)
Case 1 was refractory to CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) as well as salvage treatment with several subsequent lines of chemotherapy, and died 11 months after enrollment due to progressive lymphoma. Case 2 received dose-adjusted EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin) and achieved a partial response.
The association of PEL with HIV and HHV8 is well established, but surprisingly, is not frequently reported from SSA where both viruses are endemic. Classic PEL presentation includes malignant body cavity effusions without a tumor mass. Conversely, EPEL presents with lymph node masses but no cavity effusions3–7. Rarer involvement of other organs has also been reported4–9.
EPEL has been described as a highly cellular nested tumor composed of medium to large cells with variably condensed chromatin, prominent nucleoli, and abundant amphophilic cytoplasm with extensive necrosis5. Both PEL and EPEL exhibit a similar immunophenotype and genotype, as well as HHV8 positivity. Tumor cells express CD45 in >90% of cases, but lack expression of B-cell and T-cell antigens consistent with a null phenotype9. Our cases had many typical immunophenotypic features of PEL, though somewhat surprisingly both were negative for CD45, which could lead to misdiagnosis if LANA IHC is not available. Although both cases were CD30-negative, CD30 expression has been reported in a subset of cases. The possible anaplastic morphology associated with frequent aberrant expression of T-cell markers and expression of CD30 may lead to a diagnosis of anaplastic large-cell lymphoma. However, presence of HHV8 in tumor cells excludes such a diagnosis3. CD138 positivity is variable7,9.
Both cases were EBER positive by ISH and Case 1 had high plasma EBV and HHV8 loads. Coinfection of tumor cells by HHV8 and EBV is highly specific to PEL/EPEL. HHV8 load is useful in evaluating patients with PEL, but neither baseline EBV nor HHV8 plasma loads are prognostic in PEL. However, with ART and chemotherapy, EBV-positive PEL has been reported to have a better prognosis than EBV-negative PEL10. Unlike HHV8, EBV is not essential to PEL development8,12.
HIV-associated EPEL, like PEL, is usually treated with CHOP-like chemotherapy plus ART17. However, it is often chemotherapy-resistant with poor survival. More intensive, continuous infusion chemotherapy regimens may be more effective than conventional CHOP13, and outcomes may be better in EPEL compared to PEL7.
Although HIV and HHV8 are highly prevalent in SSA, we found scarce reports of PEL from the region, likely reflecting under-diagnosis. Through sustained local laboratory capacity building including telepathology, we recently confirmed two EPEL cases from Malawi. We hope this report will increase awareness, and emphasize the need for improved diagnosis and treatment for this rare, likely under-recognized lymphoma subtype.
Acknowledgments
Funding: This work was supported by grants from the National Institutes of Health (K01TW009488, R21CA180815, and U54CA190152 to S.G.), the Medical Education Partnership Initiative (U2GPS001965), the Lineberger Comprehensive Cancer Center (P30CA016086), and the UNC Center for AIDS Research (P30AI50410).
References
- 1.Pan Z, Zhang Q, Lu ZJ, et al. Extracavitary KSHV-associated large B-Cell lymphoma: a distinct entity or a subtype of primary effusion lymphoma? Study of 9 cases and review of an additional 43 cases. Am J Surg Pathol. 2012;36(8):1129–1140. doi: 10.1097/PAS.0b013e31825b38ec [DOI] [PubMed] [Google Scholar]
- 2.Tumwine LK, Lalitha R, Agostinelli C, et al. Primary effusion lymphoma associated with Human Herpes Virus-8 and Epstein Barr virus in an HIV-infected woman from Kampala, Uganda: A case report. J Med Case Rep. 2011;5. doi: 10.1186/1752-1947-5-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim Y, Leventaki V, Bhaijee F, Jackson CC, Medeiros LJ, Vega F. Extracavitary/solid variant of primary effusion lymphoma. Ann Diagn Pathol. 2012;16(6):441–446. doi: 10.1016/j.anndiagpath.2012.03.004 [DOI] [PubMed] [Google Scholar]
- 4.Guillet S, Gérard L, Meignin V, et al. Classic and extracavitary primary effusion lymphoma in 51 HIV-infected patients from a single institution. Am J Hematol. 2016;91(2):233–237. doi: 10.1002/ajh.24251 [DOI] [PubMed] [Google Scholar]
- 5.Epperla N, Harrington AM, Hemauer K, Shah NN. Extracavitary primary effusion lymphoma associated with hemophagocytic lymphohistiocytosis. Am J Hematol. 2016;91(11):1161–1164. doi: 10.1002/ajh.24475 [DOI] [PubMed] [Google Scholar]
- 6.Metcalf RA, Wang L, Deos PH, Chock E, Warnke RA, Natkunam Y. Extracavity primary effusion lymphoma presenting in a lymph node without lymphomatous effusions. Hum Pathol Case Reports. 2015;2(2):36–41. doi: 10.1016/j.ehpc.2014.11.012 [DOI] [Google Scholar]
- 7.Chadburn A, Hyjek E, Mathew S, Cesarman E, Said J, Knowles DM. KSHV-positive solid lymphomas represent an extra-cavitary variant of primary effusion lymphoma. Am J Surg Pathol. 2004;28(11):1401–1416. doi: 10.1097/01.pas.0000138177.10829.5c [DOI] [PubMed] [Google Scholar]
- 8.Pantanowitz L, Wu Z, Dezube BJ, Pihan G. Extracavitary primary effusion lymphoma of the anorectum. Clin Lymphoma Myeloma. 2005;6(2):149–152. doi: 10.3816/CLM.2005.n.044 [DOI] [PubMed] [Google Scholar]
- 9.Carbone A, Gloghini A. KSHV/HHV8-associated lymphomas. Br J Haematol. 2008;140(1):13–24. doi: 10.1111/j.1365-2141.2007.06879.x [DOI] [PubMed] [Google Scholar]
- 10.Lurain K, Polizzotto MN, Goncalves PH, et al. International Conference on Malignancies in HIV/AIDS. In: Tumor Epstein-Barr Virus Status Is Prognostic in Primary Effusion Lymphoma. ; 2017:28.
- 11.Boulanger E, Gérard L, Gabarre J, et al. Prognostic factors and outcome of human herpesvirus 8-associated primary effusion lymphoma in patients with AIDS. J Clin Oncol. 2005;23(19):4372–4380. doi: 10.1200/JCO.2005.07.084 [DOI] [PubMed] [Google Scholar]
- 12.Arora N, Gupta A, Sadeghi N. Primary effusion lymphoma. Curr Opin Pulm Med. 2017;23(4):365–370. doi: 10.1097/MCP.0000000000000384 [DOI] [PubMed] [Google Scholar]
- 13.Okada S, Goto H, Yotsumoto M. Current status of treatment for primary effusion lymphoma. Intractable Rare Dis Res. 2014;3(3):65–74. doi: 10.5582/irdr.2014.01010 [DOI] [PMC free article] [PubMed] [Google Scholar]


