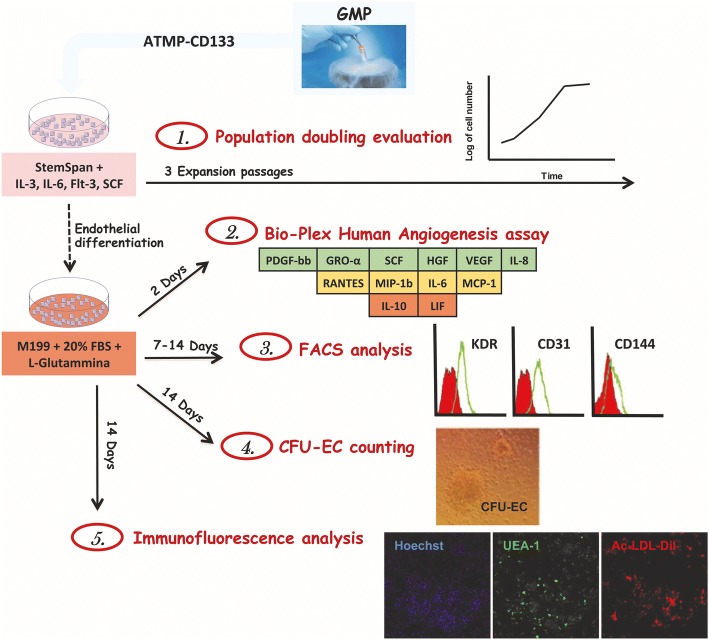
Fig. 2.
Schematic representation of the in vitro experimental plan. GMP good manufacturing practice, ATMP advanced therapy medicinal product, IL interleukin, SCF stem cell factor, FBS fetal bovine serum, PDGF-bb platelet-derived growth factor type bb, GRO-α growth-regulated oncogene alpha, HGF hepatocyte growth factor, VEGF vascular endothelial growth factor, RANTES regulated on activation normal T cell expressed and secreted, MIP-1b macrophage inflammatory protein-1 beta, MCP-1 monocyte chemoattractant protein-1, LIF leukemia inhibitory factor, FACS fluorescence-activated cell sorting, CFU-EC colony forming unit-endothelial cell, Ac-LDL-Dil acetylated low-density lipoprotein labeled with dioctadecyl-tetramethylindocarbocyanine perchlorate, UEA-1 Ulex europaeus agglutinin-1