FIG. 4.
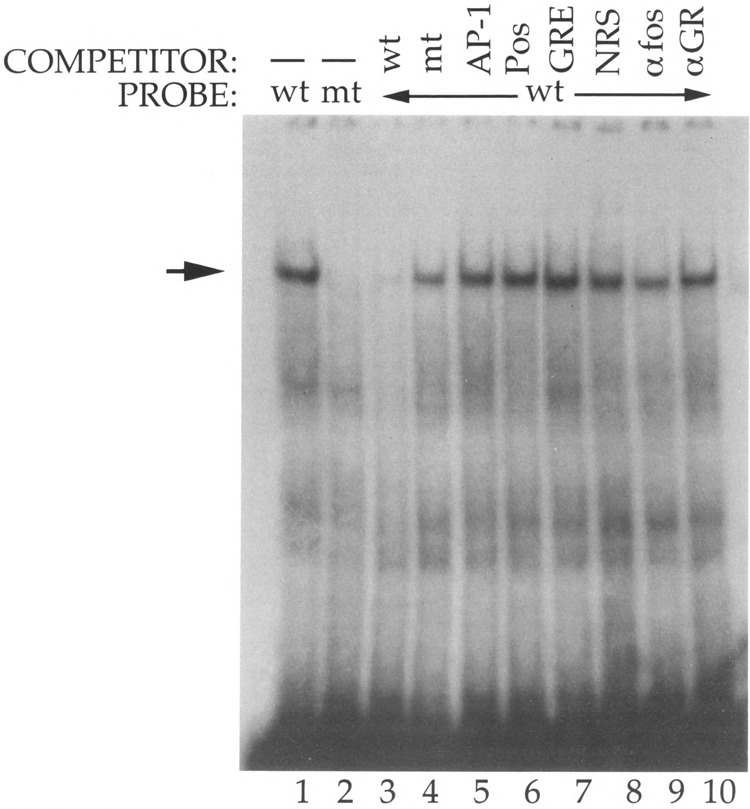
Protein binding to the cGRE/negative element. Double-stranded oligonucleotides corresponding to the wild type (lane 1) or mutant (lane 2) PLF negative element (from – 254 to – 230) were radiolabeled and incubated with mouse L cell whole-cell extracts. The mutated cGRE was altered at residues – 243 through – 234. Specific binding (arrow) was determined by competition with a 50-fold molar excess of unlabeled double-stranded wild-type (lane 3) or mutant (lane 4) negative element. Additional competition binding reactions included a 50-fold molar excess of the PLF gene AP-1 element (lane 5), the PLF positive element from – 274 to – 253 (lane 6), or five copies of the tyrosine aminotransferase GRE (lane 7). Parallel reactions were also incubated with normal rabbit serum (lane 8), an antiserum against c-fos (lane 9), or a monoclonal antibody against the glucocorticoid receptor (lane 10). Bound and free DNA were separated by polyacrylamide gel electrophoresis.
