Abstract
Knowledge on whereabouts within the annual cycle of migratory species is prerequisite for many aspects in ecology and biological conservation. Spatial assignments of stable isotopes archived in tissues allows for later inference on sites where the specific tissue had been grown. It has been rarely tested whether spatial assignments match directly tracked non-breeding residences, especially for migratory songbirds. We here compare assignments of stable isotopes from feathers of Palaearctic Barn swallows Hirundo rustica with their African non-breeding residence sites tracked by geolocation.Assignments based on δ2H, δ13C and δ15N isotope compositions delineate three main non-breeding regions: a main cluster in central Africa, a second in West Africa, and the third cluster in Northern Africa. Using δ13C, δ15N only, non-breeding sites ranged from clusters in West/Southwest Africa to South East Africa with a centre in Central Africa. The non-breeding areas (50% and 75% Kernel density estimates, KDE) of the birds tracked by geolocation stretched from West Africa via central Africa to southern Africa. We found little overlap of 0.3% (assuming a 1:1 odds ratio) to 1.4% (3:1 odds ratio) in the three element assignments and KDEs for only 2 and 13 individuals out of 32 birds. Assignment maps for two elements (δ13C, δ15N) and KDEs showed higher consistencies with an overlap of 3.6 and 8.5% for 12 and 18 birds. We argue that the low matching between stable isotope assignments and non-breeding sites in our study arise from insufficient baseline data for Africa (concerning both isoscapes and specific discrimination functions). However, other factors like aerial foraging habit of the species, and a potential mismatch of non-breeding site location and the spatial origin of aerial plankton might further hamper accurate assignments. Finally we call for concerted analyses of tissues i.e. feathers and claws of birds which are grown at known sites across the continent and from species with various ecological requirements (diverse habitats, foraging behaviours, and diet compositions) to establish isoscapes for general applicability.
Introduction
Individual residence sites outside the breeding season are still fragmentary known for many populations of migratory animals. Underlying reasons are e.g. body size constraints which impede the adoption of transmitting devices such as GSM and ARGOS PTTs, low recapture rates of individuals marked with archival tags (GPS, geolocators) [1] or even the low numbers in rare species. This lack of knowledge is unsatisfactory as migratory species are assumed to be prone to divergent changes in various environments during their annual cycle [2] and thus their actual distribution should be identified urgently to be able to track ongoing and future distributional shifts [3].
During the last decade, indirect methods for the identification of the distribution in long-distance migrants such as small passerine birds or insects became progressively more sophisticated and nowadays enable an outline of the species’ whereabouts on a very fine geographical scale [4]. The accuracy of localisation attempts seems especially crucial when targeted conservation actions subsequently are planned within the identified areas.
The analysis of naturally occurring stable isotopes archived in animal tissues is one of the most widely adopted indirect tracking method developed to date [5]. Stable isotope analyses are very powerful tools to observe various ecological phenomena in animals, related to food and water intake, metabolism and finally the incorporation of chemical elements into the animal’s tissues. Herein, the tissue’s isotopic composition mirrors the source composition of the diet in a predictable manner [6]. Moreover, various tissues in a broader sense differentially archive this information on temporal scales from hours, like the composition of breath and blood plasma [7], to very long times in metabolically inert tissues like teeth [8], keratin in claws and feathers [9,10] or in hair of ancient mummies [11].
Additionally, the distribution of many stable isotopes like δ13C, δ2H and δ18O shows distinct spatial pattern across broad geographical scales, allowing for geographical assignments of archive stable isotope compositions and thus inference on animal [12]. Today this approach is frequently used especially in the study of long-distance migratory animals whose distribution during parts of the annual cycle remains unknown so far. Prominent examples include diverse animal classes like insects [13, 14], mammals [15] and most frequently birds [16].
The approach matches the isotopic composition of a tissue, whose time of synthesis is approximately known, with the geographically specific isotope composition of the diet ingested during the focal time [17, 18]. The method, however crucially depends on contrasting geographical differences in the composition of certain elements and on detailed and complete data for ground-truthed base line maps (so called isoscapes). Unfortunately, the latter usually exist mainly for northern hemisphere regions [19]. Thus, predictions with sufficiently high confidence are possible for certain regions on Earth, whereas for other areas like the African continent such predictions might be hard to establish [20].
Assignments can be more powerful, if several chemical elements with various geographical patterns are combined. In a pioneering work, [21] proposed a detailed method for geographical assignments of Afrotropical migrant birds based on three natural stable isotopes, namely 2H, 13C and 15N, wherein the baseline modelling is done by means of a multi-isotopic cluster model. This approach was refined, among others, by [4] by applying multivariate normal probability density functions for a spatially explicit assignment of the moult origin of birds. The method is based on a high assignment resolution (e.g. 0.33° [4]) instead of the rather broad attribution to one of four or five isotopic similar regions (clusters) delineated by [21]. However, despite its increasing application, very few studies tested the assignment accuracy by matching assignment results with parallel direct tracking data such as geolocation [22, 23, 24]. To our best knowledge, the isotopic assignment of moult origins in birds in the African continent has never been previously validated using individual-based migration tracking data.
We here compared the geographical assignment to certain regions in Africa by stable isotope ratios with non-breeding residence sites determined by geolocation of widespread Palearctic–Afrotropical migrant, the Barn swallow Hirundo rustica. Barn swallows migrate from their breeding sites in Europe to sub-Saharan non-breeding sites, which range from West Africa to South Africa depending on the specific population [25, 26, 27]. Thus, the species occupies regions with very different habitats and contrasting isotopic conditions [28]. Barn swallows usually arrive in the non-breeding grounds at the end of September/beginning of October, where they remain stationary for about six months until spring departure [29]. At their non-breeding sites, adult Barn swallows accomplish the moult of wing feathers [30]. Consequently feathers grown during the non-breeding period within Africa should reflect the stable isotope composition of prey and water available at these particular non-breeding sites and within a confined period (e.g. growing season). In our study, we tested the hypothesis that regions derived from spatial assignment of stable isotope composition of African-grown feathers match the African non-breeding sites derived by geolocator tracking.
Material and methods
Study system and geographical assignment by light-level geolocation
We used data from a geolocation study on the non-breeding distribution of individual Barn swallows breeding in southern Switzerland (about 46°N, 9°E) and northern Italy (about 45°N, 9°E) [29]. Birds had been equipped with geolocators (type SOI-GDL2, Swiss Ornithological Institute) in spring 2010 and 2011. After spring arrival in the subsequent year, geolocators were collected and a feather sample from the wing (the innermost tertial) of returning birds at their respective breeding sites (for details on the geolocator study see [31, 29]). Non-breeding sites were distributed in sub-Saharan Africa from Mali and Senegal in the West to South Africa; the main residences were located in the region of Cameroon to Nigeria where about 88% of the studied individuals overwintered in a 1000 km wide area [29].
For the matching of isotope assignments and geolocation, we used a subset of 32 birds (22 males, 10 females, from 2010/11 and 4 from 2011/12), which represent the entire non-breeding range with three individuals each for West and southern Africa) as well as a random selection of 26 birds for the main nonbreeding grounds for this studied population (see above and Fig 1). The individual non-breeding areas were determined by 50% and 75% kernel density estimations (KDE) (300 km search radius, ArcGIS 9.3) using geolocator location during the nonbreeding period, i.e. after arrival in the beginning of October and before departure in the beginning of March from sub-Saharan nonbreeding sites. Wing moult in barn swallows is usually performed entirely during the non-breeding period [30], thus we assume the wing feather had been completely grown at these non-breeding sites.
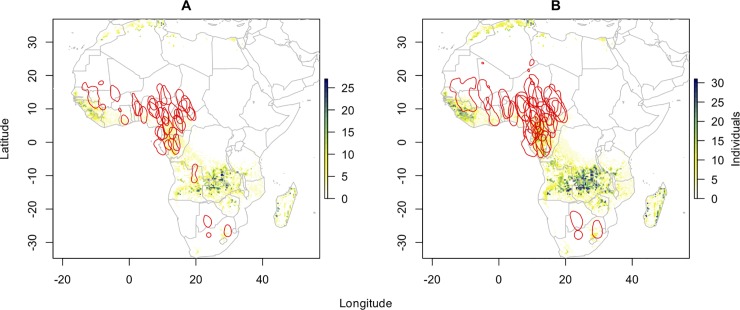
Fig 1. Predicted moult origin/nonbreeding regions for barn swallows based on isotope assignment for three isotopes δ2H, δ13C and δ15N and geolocation.
Kernel density estimates are shown in red. The colours indicate the number of individuals that were isotopically consistent with a given raster cell in the isoscape representing the likely moulting site. a) Odd ratio 1:1 and 50% KDE. b) Odd ratio 3:1 and 75% KDE.
Stable isotope analysis
The feather samples were cleaned with hexane to remove contaminations, and subsequently air-dried under a fume hood in the laboratory. For δ13C and δ15N determination, about 0.3 mg of the feather vane samples were weighed into tin capsules. Their determination in one run was carried out using an isotope ratio mass spectrometer (Isoprime, Elementar Analysensysteme GmbH, Germany) interfaced with an elemental analyser (Vario Isotope Cube, Elementar Analysensysteme GmbH, Germany). For the analysis of δ2H, about 0.2 mg of sample was placed in silver capsules and, once weighed, the samples and reference materials were left in laboratory air moisture for at least 96 h, then placed in a desiccator with P2O5 under vacuum for a further 96 h. Samples were then loaded onto the autosampler tray, put on the carousel, sealed with a cover and purged with argon. δ2H was determined using an isotope ratio mass spectrometer equipped with a TC/EA (thermo combustion pyrolyser—elemental analyser; Delta Plus XP -ThermoFinnigan, Bremen, Germany).
The isotope ratios were expressed in δ against V-PDB (Vienna—Pee Dee Belemnite) for δ13C, Air for δ15N and V-SMOW (Vienna—Standard Mean Ocean Water) for δ2H according to [32]. The values of δ2H were calculated building a regression line through the two reference materials Caribou Hoof Standard (CHS, USGS—United States Geological Survey, Reston Stable Isotope Laboratory, Virginia, USA) and Kudu Horn Standard (KHS, USGS). The reference values of the reference materials considered were δ2H = -197.0 ‰ for CBS and δ2H = -54.1 ‰ for KHS. Therefore, the δ2H values of the samples were expressed in comparison to V-SMOW on scales normalized in such a way that the δ2H value of SLAP (Standard Light Antarctic Precipitation) was -428 ‰, as recommended by IUPAC [33]. In each analytical sequence, analysis of an internal quality control material (keratin, Camida Ltd., Tipperary, Ireland) was included to check analytical system performance.
δ13C and δ15N isotopic values were calculated against in-house standards, which were themselves calibrated against international reference materials: fuel oil NBS-22 (IAEA International Atomic Energy Agency, Vienna, Austria; -30.031 ‰) and sugar IAEA-CH-6 (-10.449 ‰) for δ13C, L-glutamic acid USGS 40 (-26.389 ‰ and -4.5 ‰ for δ13C and δ15N), hair USGS 42 (δ15N = +8.05 ‰ and δ13C = -21.09 ‰) and USGS 43 (δ15N = +8.44 ‰ and δ13C = -21.28 ‰) for 13C/12C and 15N/14N.
Method uncertainty (calculated as one standard deviation in repeatability conditions) was 0.1 ‰ for δ13C, 0.2 ‰ for δ15N and 2 ‰ for δ2H.
Geographic assignment to the moult origin by stable isotopes
We aimed at identifying the moulting area during the nonbreeding period by applying a multiple element approach [21, 4]. We used feather isotope data of δ13C, δ15N and δ2H with a resolution 0.33° to perform spatially explicit assignments to isoscapes of Africa. The feather isoscapes were derived from 1) isoscapes of amount-weighted mean growing season δ2H in precipitation (δ2Hp, [34]), 2) the theoretical spatial δ13C distribution of plants [35] and 3) plant δ15N isoscape developed by [36], following the method developed by [21]. Thereby, the δ2Hp isoscape (p-precipitation) was converted into a δ2Hf isoscape (f-feather) based on regression parameters derived from a regression of δ2Hf in feathers of insectivorous Eurasian reed warblers (Acrocephalus scirpaceus) against δ2Hp [37]. Discrimination between plant and feather δ13C and δ15N isoscapes were accounted for by a discrimination factor of +2‰ for δ13C and +5‰ for δ15N [21].
We determined the likelihood that a given raster cell within the feather isoscapes represents a potential moult origin of a particular feather by applying multivariate normal probability density functions (mvnpdf) following [4]. Thereby, we firstly considered the set of three isotopes δ13C, δ15N and δ2H, and then we repeated the procedure based only on the set of two isotopes δ13C and δ15N resulting in two different probability surfaces for every individual. We did not include the species’ known nonbreeding distribution as prior information in the assignment procedure.
In a second step, we reclassified each raster cell into likely (1) and unlikely (0) by selecting those geographic locations that fell within the upper 50% (1:1 odd ratio) and 75% (3:1 odd ratio) of the spatially explicit probability densities within the likelihood map. These values were chosen as they correspond to the 50% and 75% KDE of the geolocator locations. The resulting individual binary assignment maps were summed up to depict the likely population moult origin.
Furthermore, in order to test the applicability of the isotopic clusters proposed by [21] we estimated the assignment of individual feather stable isotope (SI) values to four and five isotopic clusters (identified by [21]) derived by cluster analysis based on δ13C, δ15N and δ2H or δ13C and δ15N, respectively. In this assignment procedure, linear discriminant function analysis (DFA) is used to predict the posterior probability that a sample with a given multi-isotope composition could have originated from any given cluster within Africa, given the predicted ranges (min, max, mean and SD) for feather δ13C, δ15N and δ2H [21].
All assignments were performed by using the “mvnmle”, “raster” and “maptools” packages [38, 39, 40] in R 3.1 [41].
Matching geographical assignment by stable isotopes and geolocation
We quantified the overlap of isotopic assignment and geolocation by calculating the percentage of raster cells of likely moult origin (by stable isotopes) overlapping with the individual 50% and 75% KDE derived from geolocation.
In addition, we calculated the percentage of cells within 50% and 75% KDE assigned to the four (δ13C, δ15N and δ2H) and five (δ13C, δ15N) isotopic clusters based on the cluster analysis by [21].
This study was carried out under permission #301 of Progetto Rondine Sul Piano di Magadino, Italy. All efforts were made to minimize handling time of the birds while geolocators were attached and removed.
Results
Identification of nonbreeding areas by geolocation
The non-breeding areas of the subset of Barn swallows used in this study stretched from 5°W to 31°E longitude and 15°N to 28°S latitude (Fig 1, for the complete data set see [29]) including a variety of main vegetation types from grass savannah (West Africa), tree savannah and tropical forest (eastern west and central Africa) to mainly steppe habitats (southern Africa). The median area of non-breeding sites calculated as kernel density area comprised about 149,020 km2 (25–75%: 111,495–171,191 km2) for the 50% KDE and 345,730 km2 (25–75%: 255,200–418,764 km2) for the 75% KDE. This corresponds to approximately 107 and 252 cells for the stable isotope assignment (see below).
Geographical assignment by stable isotopes
Assignment based on stable isotope composition of three elements, i.e. δ2H, δ13C and δ15N highlighted four main regions comprising the likely moult origins (Fig 1A and 1B). For 31 of 32 individuals, origins were located in raster cells south of 5°S, in DR Congo, Zambia, Angola and neighbouring countries as well as Madagascar. Furthermore, a third cluster of likely moult origin was found in West Africa (Guinea Bissau—Liberia), while a third cluster comprised cells close to the coastlines in Northern Africa (Morocco, Tunisia and the Nile delta in Egypt). On average a likely moult origin was assigned for 414 cells (1:1 odds) and for 953 cells (3:1 odds; Table 1).
Table 1. Overview of geographical position (Latitude and Longitude), size of nonbreeding range of Barn swallows (KDE-Kernel density estimates in km2 and number of raster cells), the number of assigned raster cells based on stable isotope assignments (CN = two isotopes: δ2H, δ13C and HCN = three isotopes δ2H, δ13C and δ15N) and overlap of matching raster cells (KDE & SI assignment, the number of cells and %).
| ID | Lat (°) | Long (°) | Size KDE (km2) | Size KDE (# cells) | Assignment based on CN (# cells) | Assignment based on HCN (# cells) | Overlap KDE and assignments HCN (# of cells, %) | Overlap KDE and assignments CN (# of cells, %) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 % | 75 % | 50 % | 75 % | odds 1:1 | odds 3:1 | odds 1:1 | odds 3:1 | 50 % | 75 % | 50 % | 75 % | 50 % | 75 % | 50 % | 75 % | |||
| 1RZ | 4.0 | 14.6 | 171826 | 348808 | 133 | 269 | 828 | 1420 | 312 | 813 | 0.0 | 3.0 | 0.0 | 1.1 | 6.0 | 66.0 | 4.5 | 24.5 |
| 1ST | 9.2 | -2.9 | 127633 | 281490 | 98 | 217 | 1671 | 3143 | 770 | 1578 | 0.0 | 1.0 | 0.0 | 0.5 | 0.0 | 11.0 | 0.0 | 5.1 |
| 1SU | -0.4 | 14.2 | 133563 | 268400 | 103 | 207 | 866 | 1824 | 400 | 1040 | 0.0 | 21.0 | 0.0 | 10.1 | 7.0 | 71.0 | 6.8 | 34.3 |
| 1TQ | 13.5 | 10.3 | 183776 | 444172 | 142 | 343 | 1073 | 1771 | 415 | 1006 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 1TS | 12.6 | -8.0 | 124394 | 324231 | 96 | 250 | 341 | 1093 | 202 | 726 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 1UE | 9.5 | 7.4 | 91977 | 201018 | 71 | 155 | 920 | 1556 | 405 | 1011 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 1UH | 8.6 | 6.6 | 139026 | 329324 | 107 | 254 | 1169 | 2004 | 194 | 462 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 1UJ | 10.2 | 16.0 | 269300 | 522239 | 208 | 403 | 1147 | 1931 | 560 | 1241 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 1UY | 10.1 | 17.1 | 278742 | 551148 | 215 | 425 | 1021 | 1688 | 584 | 1160 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 1WG | 6.4 | 10.5 | 155518 | 322219 | 120 | 249 | 914 | 1541 | 199 | 464 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 5.0 | 0.0 | 2.0 |
| 1WH | 0.9 | 13.0 | 102288 | 297575 | 79 | 230 | 1636 | 3434 | 620 | 1289 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 21.0 | 0.0 | 9.1 |
| 1WW | 1.5 | 8.7 | 139341 | 458723 | 108 | 354 | 1031 | 1789 | 175 | 414 | 0.0 | 0.0 | 0.0 | 0.0 | 17.0 | 84.0 | 15.8 | 23.7 |
| 1XR | 7.0 | 10.6 | 115981 | 323817 | 89 | 250 | 866 | 1466 | 197 | 515 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 1XT | 3.6 | 11.5 | 171685 | 344246 | 132 | 266 | 845 | 1494 | 225 | 626 | 0.0 | 3.0 | 0.0 | 1.1 | 14.0 | 63.0 | 10.6 | 23.7 |
| 1YA | -1.3 | 8.9 | 173642 | 420043 | 134 | 324 | 1295 | 2526 | 647 | 1363 | 0.0 | 12.0 | 0.0 | 3.7 | 18.0 | 78.0 | 13.4 | 24.1 |
| 1YD | -27.9 | 25.6 | 125560 | 233820 | 97 | 180 | 1401 | 2684 | 929 | 1899 | 7.0 | 2.0 | 7.2 | 1.1 | 4.0 | 1.0 | 4.1 | 0.6 |
| 1YW | 7.7 | 7.3 | 202535 | 414928 | 156 | 320 | 166 | 855 | 113 | 507 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 1ZS | 0.6 | 6.3 | 101523 | 242870 | 78 | 187 | 996 | 1710 | 227 | 570 | 0.0 | 0.0 | 0.0 | 0.0 | 8.0 | 37.0 | 10.2 | 19.7 |
| 1ZV | 3.3 | 15.6 | 160011 | 307053 | 123 | 237 | 790 | 1397 | 267 | 748 | 0.0 | 0.0 | 0.0 | 0.0 | 3.0 | 27.0 | 2.4 | 11.4 |
| 2AA | 12.7 | 6.2 | 157142 | 329983 | 121 | 255 | 1114 | 1934 | 110 | 282 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 2AI | 13.1 | -10.4 | 294337 | 631533 | 227 | 487 | 592 | 1349 | 201 | 520 | 0.0 | 11.0 | 0.0 | 2.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| 2AL | 7.6 | 13.2 | 147322 | 456900 | 114 | 353 | 1177 | 2001 | 470 | 1055 | 0.0 | 3.0 | 0.0 | 0.9 | 0.0 | 1.0 | 0.0 | 0.3 |
| 2AR | 6.3 | 9.1 | 198118 | 404295 | 153 | 312 | 134 | 888 | 148 | 592 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 0.0 | 0.3 |
| 2AZ | 9.1 | 15.9 | 111478 | 212401 | 86 | 164 | 1253 | 2321 | 643 | 1377 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 2BJ | 7.4 | -0.3 | 111544 | 220143 | 86 | 170 | 968 | 1662 | 225 | 476 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 2CT | 5.4 | 7.8 | 124339 | 323513 | 96 | 250 | 909 | 1537 | 407 | 963 | 2.0 | 16.0 | 2.1 | 6.4 | 1.0 | 6.0 | 1.0 | 2.4 |
| 2DC | -3.6 | 14.0 | 149296 | 340756 | 115 | 263 | 1042 | 2327 | 523 | 1194 | 0.0 | 17.0 | 0.0 | 6.5 | 16.0 | 92.0 | 13.9 | 35.0 |
| 2DF | 7.2 | 18.4 | 156047 | 498641 | 120 | 385 | 1189 | 2082 | 138 | 336 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 0.0 | 0.3 |
| 3CX | 4.9 | -4.5 | 60807 | 210245 | 47 | 162 | 767 | 1374 | 464 | 975 | 0.0 | 1.0 | 0.0 | 0.6 | 0.0 | 0.0 | 0.0 | 0.0 |
| 3RD | -2.8 | 14.5 | 95104 | 343880 | 73 | 265 | 988 | 2200 | 437 | 1043 | 0.0 | 14.0 | 0.0 | 5.3 | 20.0 | 114.0 | 27.3 | 43.0 |
| 3RN | -25.0 | 20.6 | 105973 | 250801 | 82 | 194 | 1567 | 3235 | 1142 | 2412 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 3ST | 7.9 | -2.7 | 96932 | 204126 | 75 | 158 | 1877 | 3448 | 915 | 1841 | 0.0 | 9.0 | 0.0 | 5.7 | 5.0 | 20.0 | 6.7 | 12.7 |
| mean | 149274 | 345729 | 115 | 267 | 1017 | 1928 | 415 | 953 | 0.3 | 3.5 | 0.3 | 1.4 | 3.7 | 21.8 | 3.6 | 8.5 | ||
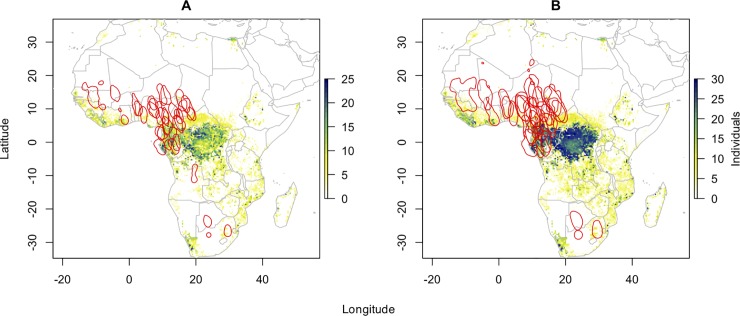
Assignments derived by two stable isotopes, i.e. δ13C and δ15N, were less distinct with a mean number of 1017 assigned cells for the 1:1 odds and 1928 assigned cells for 3:1 odds (Table 1). Herein, likely moult origins ranged from a cluster in sub-Saharan West Africa to South West and South East Africa with a clear agglomeration of individual assignments for Central Africa (Cameroon, DR Congo, Central African Republic). A small cluster again was located in the Nile delta (Fig 2A and 2B).
Fig 2. Predicted moult range/nonbreeding range for barn swallows based on isotope assignment for two isotopes δ13C and δ15N and geolocation.
The different colours of raster cells indicate the number of individuals that were isotopically associated with a given raster cell of the isoscape. Kernel density estimates (KDE) derived from geolocation are shown in red. a) Odd ratio 1:1 and 50% KDE. b) Odd ratio 3:1 and 75% KDE.
The discriminant function analysis predominantly assigned feather isotope values to isotopic cluster 1 and 2 based on three isotopes δ2H, δ13C and δ15N with an average likelihood of 0.89 (Table 2). Considering only δ13C and δ15N, the bulk of individuals was clearly assigned to cluster 3 with only five individuals assigned to cluster 2. Average likelihood amounted to 0.99 (Table 3).
Table 2. Assignment of individual feather stable isotope value to four isotopic clusters based on δ2H, δ13C and δ15N (according to [21]) and the percentage of raster cells assigned to four isotopic clusters within the individual 50% KDE and 75% KDE.
Grey shading indicates corresponding assignment of individual feather stable isotopes and KDE.
| Assignment Isotopes | Cells within 50% KDE | Cells within 75% KDE | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SampleID | C1 | C2 | C3 | C4 | C1 | C2 | C3 | C4 | C1 | C2 | C3 | C4 |
| 1RZ | 100 | 0 | 0 | 0 | 23 | 76 | 1 | 0 | 41 | 52 | 8 | 0 |
| 1ST | 0 | 100 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 98 | 2 |
| 1SU | 4 | 96 | 0 | 0 | 73 | 27 | 0 | 0 | 76 | 24 | 0 | 0 |
| 1TQ | 88 | 12 | 0 | 0 | 0 | 0 | 61 | 39 | 0 | 0 | 65 | 35 |
| 1TS | 98 | 2 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 |
| 1UE | 100 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 2 | 98 | 0 |
| 1UH | 40 | 60 | 0 | 0 | 0 | 6 | 94 | 0 | 0 | 6 | 94 | 0 |
| 1UJ | 78 | 22 | 0 | 0 | 5 | 61 | 34 | 0 | 4 | 57 | 39 | 0 |
| 1UY | 100 | 0 | 0 | 0 | 0 | 0 | 81 | 19 | 4 | 73 | 23 | 0 |
| 1WG | 100 | 0 | 0 | 0 | 9 | 32 | 60 | 0 | 10 | 29 | 62 | 0 |
| 1WH | 0 | 100 | 0 | 0 | 97 | 3 | 0 | 0 | 73 | 26 | 1 | 0 |
| 1WW | 32 | 68 | 0 | 0 | 91 | 9 | 0 | 0 | 57 | 21 | 21 | 0 |
| 1XR | 100 | 0 | 0 | 0 | 2 | 48 | 51 | 0 | 1 | 28 | 72 | 0 |
| 1XT | 86 | 14 | 0 | 0 | 57 | 39 | 4 | 0 | 54 | 28 | 18 | 0 |
| 1YA | 0 | 100 | 0 | 0 | 89 | 5 | 5 | 0 | 89 | 8 | 3 | 0 |
| 1YD | 0 | 100 | 0 | 0 | 41 | 58 | 1 | 0 | 29 | 64 | 7 | 0 |
| 1YW | 100 | 0 | 0 | 0 | 0 | 4 | 96 | 0 | 0 | 5 | 95 | 0 |
| 1ZS | 52 | 48 | 0 | 0 | 88 | 12 | 0 | 0 | 79 | 21 | 0 | 0 |
| 1ZV | 100 | 0 | 0 | 0 | 15 | 83 | 2 | 0 | 22 | 61 | 17 | 0 |
| 2AA | 34 | 56 | 0 | 0 | 0 | 0 | 79 | 21 | 0 | 0 | 73 | 27 |
| 2AI | 56 | 44 | 0 | 0 | 0 | 22 | 78 | 0 | 0 | 23 | 75 | 2 |
| 2AL | 12 | 88 | 0 | 0 | 0 | 13 | 87 | 0 | 0 | 15 | 68 | 17 |
| 2AR | 88 | 12 | 0 | 0 | 12 | 18 | 69 | 0 | 17 | 15 | 68 | 0 |
| 2AZ | 12 | 88 | 0 | 0 | 0 | 0 | 93 | 7 | 0 | 0 | 84 | 16 |
| 2BJ | 100 | 0 | 0 | 0 | 1 | 4 | 94 | 0 | 2 | 4 | 94 | 0 |
| 2CT | 100 | 0 | 0 | 0 | 15 | 34 | 51 | 0 | 12 | 27 | 62 | 0 |
| 2DC | 0 | 100 | 0 | 0 | 73 | 27 | 0 | 0 | 76 | 23 | 1 | 0 |
| 2DF | 2 | 98 | 0 | 0 | 1 | 1 | 98 | 0 | 16 | 13 | 70 | 1 |
| 3CX | 100 | 0 | 0 | 0 | 20 | 2 | 78 | 0 | 9 | 1 | 89 | 1 |
| 3RD | 0 | 100 | 0 | 0 | 74 | 26 | 0 | 0 | 68 | 21 | 11 | 0 |
| 3RN | 0 | 100 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 |
| 3ST | 0 | 100 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 |
Table 3. a) Assignment of individual feather SI value to five isotopic clusters based on δ13C and δ15N (according to [21]) and percentage of raster cells assigned to four isotopic clusters within individual 50% and 75% KDE.
Grey shading indicates corresponding assignment of individual feather stable isotopes and KDE.
| Assignment Isotopes | Cells within 50% KDE | Cells within 75% KDE | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SampleID | C1 | C2 | C3 | C4 | C5 | C1 | C2 | C3 | C4 | C5 | C1 | C2 | C3 | C4 | C5 |
| 1RZ | 0 | 0 | 100 | 0 | 0 | 0 | 78 | 22 | 0 | 0 | 0 | 58 | 39 | 4 | 0 |
| 1ST | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 59 | 41 | 0 | 0 | 0 | 51 | 49 |
| 1SU | 0 | 0 | 100 | 0 | 0 | 0 | 30 | 70 | 0 | 0 | 0 | 26 | 74 | 0 | 0 |
| 1TQ | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 12 | 88 | 0 | 0 | 0 | 21 | 79 |
| 1TS | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 1 | 99 | 0 | 0 | 0 | 24 | 76 |
| 1UE | 0 | 0 | 100 | 0 | 0 | 0 | 3 | 0 | 97 | 0 | 0 | 3 | 0 | 79 | 18 |
| 1UH | 0 | 0 | 100 | 0 | 0 | 0 | 6 | 0 | 94 | 0 | 0 | 7 | 0 | 83 | 10 |
| 1UJ | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 5 | 31 | 63 | 0 | 0 | 4 | 33 | 63 |
| 1UY | 0 | 0 | 100 | 0 | 0 | 0 | 11 | 0 | 53 | 36 | 0 | 15 | 1 | 47 | 37 |
| 1WG | 0 | 0 | 100 | 0 | 0 | 0 | 39 | 7 | 53 | 0 | 1 | 32 | 10 | 58 | 0 |
| 1WH | 0 | 100 | 0 | 0 | 0 | 0 | 2 | 98 | 0 | 0 | 0 | 25 | 75 | 0 | 0 |
| 1WW | 0 | 0 | 100 | 0 | 0 | 3 | 14 | 83 | 0 | 0 | 5 | 30 | 50 | 14 | 0 |
| 1XR | 0 | 0 | 100 | 0 | 0 | 0 | 63 | 0 | 37 | 0 | 0 | 36 | 0 | 44 | 21 |
| 1XT | 0 | 0 | 100 | 0 | 0 | 1 | 37 | 60 | 2 | 0 | 3 | 29 | 53 | 15 | 0 |
| 1YA | 0 | 18 | 82 | 0 | 0 | 0 | 21 | 79 | 0 | 0 | 1 | 15 | 84 | 0 | 0 |
| 1YD | 0 | 100 | 0 | 0 | 0 | 38 | 59 | 3 | 0 | 0 | 63 | 33 | 3 | 1 | 0 |
| 1YW | 0 | 0 | 100 | 0 | 0 | 0 | 4 | 0 | 85 | 10 | 0 | 7 | 0 | 72 | 21 |
| 1ZS | 0 | 0 | 100 | 0 | 0 | 8 | 20 | 72 | 0 | 0 | 12 | 24 | 63 | 0 | 0 |
| 1ZV | 0 | 0 | 100 | 0 | 0 | 0 | 90 | 10 | 0 | 0 | 0 | 77 | 18 | 5 | 0 |
| 2AA | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 21 | 79 | 0 | 0 | 0 | 26 | 74 |
| 2AI | 0 | 0 | 100 | 0 | 0 | 0 | 16 | 0 | 36 | 47 | 0 | 20 | 0 | 32 | 48 |
| 2AL | 0 | 0 | 100 | 0 | 0 | 0 | 17 | 0 | 77 | 6 | 0 | 18 | 0 | 41 | 40 |
| 2AR | 0 | 0 | 100 | 0 | 0 | 4 | 20 | 9 | 67 | 0 | 4 | 20 | 11 | 59 | 6 |
| 2AZ | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 45 | 55 | 0 | 0 | 0 | 45 | 55 |
| 2BJ | 2 | 0 | 98 | 0 | 0 | 0 | 30 | 0 | 70 | 0 | 0 | 21 | 2 | 73 | 4 |
| 2CT | 0 | 0 | 100 | 0 | 0 | 0 | 41 | 15 | 44 | 0 | 1 | 30 | 12 | 48 | 9 |
| 2DC | 0 | 18 | 82 | 0 | 0 | 0 | 30 | 70 | 0 | 0 | 0 | 25 | 75 | 0 | 0 |
| 2DF | 0 | 0 | 100 | 0 | 0 | 0 | 26 | 0 | 73 | 1 | 0 | 31 | 0 | 55 | 13 |
| 3CX | 0 | 0 | 100 | 0 | 0 | 0 | 38 | 14 | 48 | 0 | 0 | 17 | 8 | 69 | 6 |
| 3RD | 0 | 0 | 100 | 0 | 0 | 0 | 26 | 74 | 0 | 0 | 0 | 29 | 66 | 5 | 0 |
| 3RN | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 100 | 0 |
| 3ST | 0 | 100 | 0 | 0 | 0 | 0 | 2 | 0 | 92 | 6 | 0 | 1 | 0 | 69 | 30 |
Matching stable isotope assignment with geolocation
We found little overlap of stable isotope assignments based on δ2H, δ13C and δ15N and nonbreeding areas calculated as by KDE. Overlap increased when considering larger nonbreeding areas: For the 1:1 odds corresponding to the 50% KDE we found a match for only 2 individuals, whereas for the 3:1 odds and the 75% KDE 13 individuals (40%) showed some overlap (Table 1, S1 Fig).
However, the overlap between KDE and assigned cells was on average 0.3% for the 1:1 odds and 1.4% for the 3:1 odds (Table 1).
The assignments based on two isotopes (δ13C and δ15N), individual KDEs and isotope assignment maps were more consistent: 12 and 18 KDE comprised likely raster cells with a mean overlap of 3.6 and 8.5% (Table 1).
Similar to the little overlap between KDEs and isotope assignments based on 0.33° cells, there was hardly any consistency in the individual assignment to isotopic clusters and the cluster(s) represented by the cells inside the KDEs (Table 2). For the three-element cluster approach, only for three (50% KDEs) and two (75% KDEs) individuals, the assigned isotopic cluster corresponded with the cluster categories of cells inside the KDEs (with > 50% of raster cells representing one cluster; Table 2). For the two-element cluster approach (δ13C and δ15N) we found more matches between cluster assignments and cells within the KDEs: for eight (50% KDE) and seven individuals (75% KDE) clusters were the same for both methods (Table 3).
Discussion
Our study highlighted a lack of concordance between the individuals’ assignment based on δ2H, δ13C and δ15N isotopes and the corresponding non-breeding sites derived from geolocation for Barn swallows during their non-breeding period in Africa.
This mismatch could be ascribed to several ecological as well methodological reasons. The expectation on a similarity of SI assignment and geolocation is based on some fundamental assumptions: (1) the geolocation kernel density estimate represents the bird‘s non-breeding residence site (during boreal winter), (2) the study species moult their wing feathers in their African non-breeding grounds. Further, (3), the diet ingested by swallows originates from the specific non-breeding site tracked by geolocators and finally (4) the isotopic composition of local diet is adequately reflected by the isoscape model. Each of these assumptions will be discussed below.
It is generally accepted that geolocation by light correctly determines locations based on predictive differences in sun rise and sun set times. However, the method has inherent inaccuracies caused by environmental shading and the behaviour of the tagged individual during sun rise/sun set times [42]. Generally, estimates of latitude are more inaccurate than longitude estimates, and inaccuracy is largest during equinox periods [43, 44]. In our study, we determined residence sites of Barn swallows during the boreal winter, and thus uncertainties due to equinox can be excluded. Moreover we used a rather conservative approach (KDE) for determining non-breeding areas, which may even most likely overestimate a Barn swallow’s home range during the non-breeding period. Thus, we would expect an overestimation in the overlap between non-breeding sites and SI assignment per individual instead of the general low percentage of matched raster cells per individual found in this study.
Correct spatial assignments based on stable isotope composition of e.g. feather keratin require prior knowledge about the time of formation of the targeted tissue. Barn swallows which moult outside the non-breeding season, e.g. at the end of the breeding season or en route during migration will certainly cause faulty assignments. Although there are some observations of wing moult had been already started on the European breeding grounds [45], the proportion of these early moulting birds in a population is generally very low with about 3% for Central European breeders [45]. Applying this percentage to our study, we would expect that not more than a single bird would carry a non-African isotope signature in its tertial wing feathers. Moreover, we can exclude the possibility that we simply could have missed the birds’ moulting period and thus site, as wing feather moult usually extents over almost the entire season when birds occur in sub-Saharan Africa [46]. Even acknowledging a small proportion of birds to have moulted their tertials outside the African non-breeding distribution, we would expect that isotopic composition in feathers would allow at least for some correct assignments.
Barn swallows are aerial feeders, preying on flying insects which might disperse over larger distances by wind drift. As dispersal distances of small insects like mosquitoes and blackflies can reach several hundreds of km when transported passively by winds [47, 48], influxes of alien isotope compositions could be a likely reason which has led to the mismatch of our geolocation and SI assignment results.
However, the prevailing wind directions in SW-Africa during the non-breeding season are rather NE or SW [49], which does not support a passive transport of insects from the Congo Basin (as suggested by SI assignments) to the majority of the Barn swallow’s non-breeding sites in Cameroon and Nigeria. It cannot be entirely excluded that the diet ingested by swallows originates, to some part, from outside the specific non-breeding site. However, the lack of knowledge both in diet selection during the non-breeding period as well as the spatial extent of both passive and active movements in prey insects within sub-Saharan Africa renders a conclusion impossible.
Consequently, we speculate we can exclude a significant effect of 1) potential inaccuracy of geolocation and 2) uncertainties in the timing and location of moult and as well do not consider 3) large-scale relocation of prey as a (major) probable cause for the mismatch found in our data set.
Rather, we assume that the reason for the discrepancy between isotopic assignment and actual residence of the birds is an inadequate reflection of isotopic composition, e.g. of local diet by the available isoscape models. Firstly, inconsistencies can be attributed to a mere lack of a sufficient data base, as the accuracy of modelled isoscapes naturally highly depends on underlying data. For the African continent, base data are less comprehensive than for other region on earth. For instance, the fundamental δ2H isoscape for Africa reflecting amount-weighted mean growing season δ2H in precipitation (δ2HP, [34]) is based on data from only 44 sampling stations which are not evenly distributed across the continent and for which years of collection also vary substantially [50].
Large inner- and inter-annual variation in δ2HP caused by high fluctuations in the amount of precipitation [51, 52], and strong effects of local habitat due to evaporation [53] are further factors of uncertainty leading to an isoscape which might be too coarse for a precise delineation of individual distribution. In our data set, the limits of the available δ2H isoscape are already reflected in a relative improvement of assignment maps when we considered only δ13C and δ15N in the multivariate normal probability density functions. The overlap between KDE and isotope assignment clearly increased. However, the core areas of KDEs around 05°N and 12°E were still spatially very distant from those target regions based on δ13C and δ15N composition (core area approx. 01°S, 24°E; see further below).
Secondly, the low matching of stable isotope and geolocation data can be a matter of scale if the resolution of the available isoscapes and the size of the individual residence site deviate substantially. Although daily ranges of Barn swallows in Africa are still not known, there is indication that the species forages on a rather large scale as ring recoveries of the species within the same season encompassed distances of about 100 km. Furthermore, wide-ranging movements up to 600 km are recorded as well [54]. Accordingly, due to their potential wide-ranging aerial foraging behaviour, we would expect Barn swallows to integrate various isotopic values across the landscape much better than an e.g. rather sedentary species such as the Aquatic warbler (Acrocephalus paludicola), for which [55] recognized isotopic signatures being inappropriate markers for geographic assignments. Aquatic warblers are habitat specialists with very confined non-breeding home ranges of only a few hectares [56] the actual isotope value of a bird`s feather was interpreted to be largely determined by very small-scale foraging behaviour at a specific location rather than by large-scale isotopic gradients.
However, although the overall spatial scale reflected in the isotopic values of the individual Barn Swallows feathers should correspond to the resolution of the available isoscapes, there might still be effects of an uneven integration of isotopic composition (by the individual bird) across the landscape. This becomes especially evident when we excluded δ2H in the isotopic assignment due to uncertainties mentioned earlier. Based only on δ13C and δ15N, isotopic assignments of the majority of birds pointed to regions in the Congo basin, dominated by Guineo-Congolian evergreen and semi-deciduous rainforest [57]—and still hundreds of kilometres distant to the individual residence sites identified by geolocation.
Although the species’ ecology during the non-breeding season is fragmentarily known, authors assume Barn Swallows to primarily prefer riverbeds and wetland habitats for roosting and foraging [45, 58]. Azonal and local habitat types such as wetlands are known to harbour proportionally more C3 plants compared to the overall C3/C4 plant ratio in the predominating ecosystems [55]. This reveals isotopic values in local plants and herbivorous insects which are more similar to tropical forests than the grassland or savannah biomes identified by geolocation. Accordingly, despite the species’ rather large-scale foraging behaviour, feather isotope values could still deviate substantially from the prevailing δ13C gradients as isoscapes do not sufficiently account for such habitat effects on spatial scales below the landscape-level.
Conclusion and outlook
The analysis of multiple stable isotopes for spatial assignments is a very powerful tool to derive geographical information. The method has been successfully applied to many terrestrial species and regions world wide (Europe/Asia: [59], North America: [60], South America: [61] and is very efficient to track individuals or carry-over effects in large sample sizes allowing for robust conclusions (i.e. [62]). Based on our dataset of 32 barn swallows we could not ground-truth isotopic assignments in Africa with areas localised by direct tracking using geolocation by light. We argue that this discrepancy may mainly arise from quality of isoscapes currently available for Africa and not from a general failure. As the evolution of sophisticated statistical methods at present theoretically enables prediction of probable moult origins with a resolution of 0.33° or higher, we caution against their precipitous use as long as underlying models for isoscapes based on a weak data basis, probably reflecting a much coarser resolution. We explicitly do not object to the application of isotopic assignments for Africa in general. As the method is low-cost, both in the lab and in the field, it is well suited for surveys accepting a (at present) coarse resolution [63, 27]. For studies requiring a finer resolution, the present base data for African feather isoscape(s) needs to be improved. Recently, there are endeavours to amend δ2H isoscape(s) by e.g. the application of feather samples with spatially explicit information [64]. However, beside the use of archival samples, systematic sampling and isotopic measurements of feathers of known origin and across several years are still fundamental for the delineation of a sound feather δ2H isoscape for Africa. Accordingly, tracking studies on species with moult in Africa should be accompanied by sampling of feathers allowing for further information on the isotopic conditions in the inferred locations.
So far, almost all assignment attempts have to be based upon African δ2H isoscapes which were calibrated by a function established for Eurasian reed warblers (Acrocephalus scirpaceus) sampled in Europe [37]. The development of a calibration function explicitly based on feathers of known origin grown within Africa might improve existing δ2H isoscapes significantly. However, these fine-tuning possibilities still have to be attended by a general improvement of the resolution and spatial arrangement of the δ2Hp-sampling stations (Global Network of Isotopes in Precipitation, GNIP) across the African continent [65].
Recently, there are large efforts to improve the existing GNIP data within the continent, i.e. IAEA is mapping surface water isotope values which can results in updated isoscapes which will likely be useful for marsh associated species or birds feeding on aquatic emergent insects [66].
Finally, we would like to call for a concerted action to solve the δ2H isoscape for Africa within the near future including sampling of species with different ecological requirements.
Supporting information
75% Kernel density estimates are shown in red. a) Example of best geographical overlap. b) Example of least overlap.
(TIFF)
Acknowledgments
We thank Fondazione Bolle di Magadino for support to CS, the ‘Ente di gestione delle aree protette del Ticino e del Lago Maggiore’ for help with fieldwork and Neringa Znakovaite for help in sample processing and first analyses. Steven van Wilgenburg kindly provided R skripts and gave support in multiple isotope assignment analyses. Martins Briedis and four anonymous reviewers gave valuable comments on earlier versions of the manuscript.
Data Availability
All data files are available from the dryad digital repository database (doi:10.5061/dryad.72q5723 Data files: Seifert_et_al_Data_Barnswallows).
Funding Statement
The study was co-funded by the EU INTERREG program (project ID 15 7624065), Fondazione Cariplo (grant UNIAGI 13357 to NS), Milan Univ. (grant 2009-ATE-0015 to DR), Univ. of Milano-Bicocca (grant 2011-ATE-0272 to RA) and the German Ornithological Union (travel grant to NiS). The Swiss federal office for environment contributed financial support for the development of the data loggers (UTF-Nr. 254, 332, 363, 400). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bridge ES, Thorup K, Bowlin MS, Chilson PB, Diehl RH, Fléron RW, Hartl P, Kays R, Kelly JF, Robinson WD, Wikelski M. Technology on the move: recent and forthcoming innovations for tracking migratory birds. BioScience 2011; 61(9):689–698 [Google Scholar]
- 2.Howard C, Stephens PA, Tobias JA, Sheard C, Butchard SHM, Willis SG. Flight range, fuel load and the impact of climate change on the journeys of migrant birds. Proc. R. Soc. B. 2018; 285:20172329 10.1098/rspb.2017.2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vickery JA, Ewing SR, Smith KW, Pain DJ, Bairlein F, Skorpilova J, Gregory RD. The decline of Afro-Palaearctic migrants and an assessment of potential causes. IBIS 2014;156:1–22 [Google Scholar]
- 4.Veen T, Hjernquist MB, Van Wilgenburg SL, Hobson KA, Folmer E, Font L, Klaasen M. Identifying the African wintering grounds of hybrid flycatches using a multi-isotope (δ2H, δ13C, δ15N) assignment approach. PLoS ONE 2014;9(5):e98075 10.1371/journal.pone.0098075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hobson KA, Wassenaar LI. Tracking Animal Migration with Stable Isotopes, Vol.2 Academic Press; 2008 [Google Scholar]
- 6.DeNiro MJ, Epstein S. Influence of diet on the distribution of carbon isotopes in animals. Geochimica et Cosmochimica Acta 1978;42:495–506. [Google Scholar]
- 7.Podlesak DW, McWilloams SR, Hatch KA. Stable isotopes in breath, blood, feces and feathers can indicate intra-individual changes in the diet of migratory songbirds. Oecologia 2005;142:501–510. 10.1007/s00442-004-1737-6 [DOI] [PubMed] [Google Scholar]
- 8.Quade J, Cerling TE, Barry JC, Morgan ME, Pilbeam DR, Chivas AR et al. A 16-Ma record of paleodiet using carbon and oxygen isotopes in fossil teeth from Pakistan. Chemical Geology 1992;94:183–192. [Google Scholar]
- 9.Hahn S, Dimitrov D, Rehse S, Yohannes E, Jenni L. Avian claw morphometry and growth determine the temporal pattern of archived stable isotopes. Journal of Avian Biology 2014;45(2):202–207. [Google Scholar]
- 10.Fairhurst GD, Bond AL, Hobson KA, Ronconi RA. Feather-based measures of stable isotopes and corticosteron reveal a relationship between trophic position and physiology in a pelagic seabird over a 153-year period. Ibis 2105;157:273–283. [Google Scholar]
- 11.Knudson KJ, Peters AH, Cagigao ET. Paleodiet in the Paracas necropolis of Wari Kayan: carbon and nitrogen isotope analysis of keratin samples from the south coast of Peru. Journal of Archaeological Science 2015;55:231–243. [Google Scholar]
- 12.West JB, Bowen GJ, Dawson TE, Tu KP. Isoscapes–Understanding movement, pattern and process on earth through isotope mapping Dordrecht, Heidelberg, London, New York: Springer; 2008. [Google Scholar]
- 13.Raymon L, Vialatte A, Plantegenest M. Combination of morphometric and isotopic tools for studying spring migration dynamics in Episyrphus balteatus. Ecosphere 2014;5(7):88. [Google Scholar]
- 14.Stefanescu C, Soto DX, Talavera G, Vila R, Hobson KA. Long-distance autumn migration across the Sahara by painted lady butterflies: exploiting resource pulses in the tropical savannah. Biology Letters 2016; 12:20160561 10.1098/rsbl.2016.0561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popa-Lisseanu AG, Sörgel K, Luckner A, Wassenaar LI, Ibáñez C, Kramer-Schadt S et al. A triple-isotope approach to predict the breeding origins of european bats. PLoS One 2012;7:e30388 10.1371/journal.pone.0030388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hobson KA, Wassenaar IL. Linking breeding and wintering grounds of neotropical migrat songbirds using stable hydrogen isotopic analysis of feathers. Oecologia 1997;109:142–148. [DOI] [PubMed] [Google Scholar]
- 17.Wunder MB, Norris DR. Improved estimates of certainty in stable isotope based methods for tracking migratory animals. 2008. Ecological applications 18(2); 549–559 [DOI] [PubMed] [Google Scholar]
- 18.Wunder MB. Using isoscapes to model probability surfaces for determining geographic origins In: West J, Bowen G, Dawson T, Tu K, editors. Isoscapes. Springer, Dordrecht; 2010. pp 251–270. [Google Scholar]
- 19.IAEA/WMO. Global network of isotopes in precipitation: the GNIP Database. 2011. Available from: http://www.iaea.org/water/
- 20.Reichlin TS, Hobson KA, Van Wilgenburg SL, Schaub M, Wassenaar LI, Martin-Vivaldi M et al. Conservation through connectivity: can isotopic gradients in Africa reveal winter quarters of a migratory bird? Oecologia 2013;171: 591–600. 10.1007/s00442-012-2418-5 [DOI] [PubMed] [Google Scholar]
- 21.Hobson KA, Van Wilgenburg SL, Wassenaar LI, Powell RL, Still CJ, Craine JM. A multi-isotope (δ13C, δ15N, δ2H) feather isoscape to assign Afrotropical migrant birds to origins. Ecosphere 2012;3:1–20. [Google Scholar]
- 22.Hallworth MT, Studds CE, Sillett TS, Marra PP. Do archival light-level geolocators and stable hydrogen isotopes provide comparable estimates of breeding-ground origin? The Auk 2013;130(2):273–282. [Google Scholar]
- 23.Hobson KA, Kardynal KJ. An isotope (δ34S) filter and geolocator results constrain a dual feather isoscape (δ2H, δ13C) to identify the wintering grounds of North American Barn Swallows. The Auk 2016;133(1):86–98. [Google Scholar]
- 24.Contina A, Bridge ES, Seavy NE, Duckles JM, Kelly JF. Using geologgers to investigate bimodal isotope patterns in Painted Buntings (Passerina ciris). The Auk 2013; 130(2):265–272 [Google Scholar]
- 25.Evans KL, Waldron S, Bradbury RB. Segregation in the African wintering ranges of English and Swiss Swallow Hirundo rustica populations: a stable isotope study. Bird Study 2003;50(3):294–299. [Google Scholar]
- 26.Ambrosini R, Møller AP, Saino N. A quantitative measure of migratory connectivity. Journal of Theoretical Biology 2009;257:203–211. 10.1016/j.jtbi.2008.11.019 [DOI] [PubMed] [Google Scholar]
- 27.Von Rönn JAC, Harrod C, Bensch S, Wolf JBW. Transcontinental migratory connectivity predicts parasite prevalence in breeding populations of the European barn swallow. 2015. Journal of Evolutionary Biology; https://doi.org/10.111/jeb.12585 [DOI] [PubMed] [Google Scholar]
- 28.Bowen GJ. Isoscapes: Spatial Pattern in Isotopic Biogeochemistry. Annual Review of Earth and Planetary Sciences 2010;38:161–187 [Google Scholar]
- 29.Liechti F, Scandolara C, Rubolini D, Ambrosini R, Korner-Nievergelt K, Hahn S et al. Timing of migration and residence areas during the non-breeding period of barn swallows Hirundo rustica in relation to sex and population. Journal of Avian Biology 2015;46:254–265. [Google Scholar]
- 30.Jenni L, Winkler R. Moult and Ageing of European Passerines. Academic Press:1994 [Google Scholar]
- 31.Scandolara C, Rubolini D, Ambrosini R, Caprioli M, Hahn S, Liechti F et al. Impact of miniaturized geolocators on barn swallow Hirundo rustica fitness traits. Journal of Avian Biology 2014;45:417–423. [Google Scholar]
- 32.Brand WA, Coplen TB. Stable isotope deltas: tiny, yet robust signature in nature. Isotopes Environ Health Stud. 2012;48(3):393–409. 10.1080/10256016.2012.666977 [DOI] [PubMed] [Google Scholar]
- 33.Brand WA, Coplen TB,Vogl J, Rosner M, Prohaska T. Assessment of international reference materials for isotope-ratio analysis (IUPAC Technical Report) Pure and Applied Chemistry. USGS Publications Warehouse; 2014;425–267. [Google Scholar]
- 34.Bowen GJ, Wassenaar LI, Hobson KA. Global application of stable hydrogen and oxygen isotopes to wildlife forensics. Oecologia 2005;143:337–348. 10.1007/s00442-004-1813-y [DOI] [PubMed] [Google Scholar]
- 35.Still CJ, Berry JA, Collatz GJ, DeFries RS. Global distribution of C3 and C4 vegetation: Carbon cycle implications. Global Biochem. cycles 2003;17(1):1006. [Google Scholar]
- 36.Craine JM, Elmore AJ, Aidar MPM, Bustamante M, Dawson TE, Hobbie EA et al. Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytologist 2009;183:980–992. 10.1111/j.1469-8137.2009.02917.x [DOI] [PubMed] [Google Scholar]
- 37.Procháska P., Van Wilgenburg S.L., Neto J.M., Yosef R. and Hobson K.A. 2013. Using stable hydrogen isotopes (δ2H) and ring recoveries to trace natal origin in a Eurasian passerine with a migratory divide.–J. Avian Biol. 44;541–550. [Google Scholar]
- 38.Gross K, Bates D. mvnmle: ML estimation for multivariate normal data with missing values. 2012. R package version 0.1–11. https://cran.r-project.org/web/packages/mvnmle/index.html [Google Scholar]
- 39.Hijmans RH. raster: Geographic data analysis and modeling. 2014. R package version 2.1–49. http://CRAN.R-project.org/package=raster [Google Scholar]
- 40.Bivand R, Lewin-Koh N. maptools: Tools for reading and handling spatial objects. 2013. R package version 0.8: http://CRAN.R-project.org/package=maptools [Google Scholar]
- 41.R Development Core Team. R Foundation for Statistical Computing, Vienna, Austria; 2014
- 42.Lisovski S, Hahn S. GeoLight–processing and analysing light-based geolocator data in R.–Methods Ecol. Evol. 2012;3:1055–1059. [Google Scholar]
- 43.Fudickar AM, Wikelski M, Partecke J. Tracking migratory songbirds: accuracy of light-level loggers (geolocators) in forest habitats. Methods Ecol. Evol. 2012; 3:47–52 [Google Scholar]
- 44.Lisovski S, Hewson CM, Klaassen RHG, Korner Nievergelt F, Kristensen MW, Hahn S. Geolocation by light: accuracy and precision affected by environmental factors. Methods Ecol. Evol. 2012; 3:603–612 [Google Scholar]
- 45.Glutz von Blotzheim UN, Bauer KM. Handbuch der Vögel Mitteleuropas, Wiesbadenm AULA Verlag 1995;10:394–449. [Google Scholar]
- 46.Ginn HB, Melville DS. Moult in birds. BTO Guide 19. TRING;1983
- 47.Pedgley DE. Windborne spread of insect-transmitted diseases of animals and man. Philosophical transactions of the royal society of London. Series B, Biological Sciences 1983;302(1111):463–470. [Google Scholar]
- 48.Chapman JW, Reynolds DR, Wilson K. Long-range seasonal migration in insects: mechanisms, evolutionary drivers and ecological consequences. Ecology letters 2015;18(3):287–302. 10.1111/ele.12407 [DOI] [PubMed] [Google Scholar]
- 49.WeatherOnline. Weather maps: Expert charts Africa. 2016. Available from https://www.weatheronline.co.uk/cgi-bin/expertcharts/
- 50.Bowen G. Waterisotopes. 2016. Available from: http://wateriso.utah.edu/waterisotopes/index.html
- 51.Weischet W. Einführung in die Allgemeine Klimatologie. Stuttgart, Teubner; 1995
- 52.Lebel T, Ali A. Recent trends in the Central and Western Sahel rainfall regime (1990–2007). J Hydrol 2009;375:52–64 [Google Scholar]
- 53.Fraser KV, McKinnon EA, Diamond AW, Chavarria L. The influence of microhabitat, moisture and diet on stable-hydrogen isotope variation in a Neotropical avian food web. Journal of Tropical Ecology 2011;27:563–572. [Google Scholar]
- 54.Oatley TB. Migrant European Swallows Hirundo rustica in southern Africa: a southern perspective. Ostrich 2000;71(1–2):205–209. [Google Scholar]
- 55.Oppel S, Pain DJ, Lindsell JA, Lachmann L, Diop I, Tegetmeyer C, Donald F. High variation reduces the value of feather stable isotope ratios in identifying new wintering areas for aquatic warblers Acrocephalus paludicola in West Africa.–J. Avian Biol. 2011;42:342–354. [Google Scholar]
- 56.Arbeiter S, Tegetmeyer C. Home range and habitat use by Aquatic Warblers Acrocephalus paludicola on their wintering grounds in northwestern Senegal. Acta Ornithologica 2011;46(2):117–126. [Google Scholar]
- 57.Sayre R, Comer P, Hak J, Josse J, Bow H, Warner M et al. A new map of standardized terrestrial ecosystems of Africa. Washington, DC: Association of American Geographers; 2013. [Google Scholar]
- 58.Del Hoyo J, Elliot A, Sargatal J. Handbook of the birds of the world Volume 3 Hoatzin to auks. Barcelona: Lynx Editions; 1996. [Google Scholar]
- 59.Fox AD, Hobson KA, de Jong A, Kardynal KJ, Koehler G, Heinicke T. Flyway population delineation in Taiga Bean Geese Anser fabalis fabalis revealed by multi-element feather stable isotope analysis. 2016. IBIS. 10.1111/ibi.12417 [DOI] [Google Scholar]
- 60.Werner SJ, Hobson KA, Van Wilgenburg SL, Fischer JW. Multi-Isotopic (δ2H, δ13C, δ15N) Tracing of molt origin for Red-Winged Blackbirds associated with Agro-Ecosystems. 2016. PLoS ONE; 11(11): e0165996 10.1371/journal.pone.0165996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hobson KA, Kardynal KJ. An isotope (δ34S) filter and geolocator results constrain a dual feather isoscape (δ2H, δ13C) to identify the wintering grounds of North American Barn Swallows. 2015. The Auk; 133(1):86–98 [Google Scholar]
- 62.Lopez-Calderon C, Hobson KA, Marzal A, Balbontin J, Reviriego M, Magallanes S, Garcia-Longoria L, de Lope F, Möller AP. Environmental conditions during winter predict age- and sex-specifig differences in reproductive success of a trans-Saharan migratory bird. 2017. Scientific Reports; 7:18082 10.1038/s41598-017-18497-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hobson KA, Möller AP, Van Wilgenburg SL. A multi-isotope (δ13C, δ15N, δ2H) approach to connecting European breeding and African wintering populations of barn swallow (Hirundo rustica). Animal Migration 2013;8–22. [Google Scholar]
- 64.Gutiérrez-Expósito C, Ramirez F, Afan I, Forero MG, Hobson KA. Toward a deuterium feather isoscape for sub-Saharan Africa: Progress, challenges and the path ahead. PLoS ONE 2015;10(9):e0135938 10.1371/journal.pone.0135938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vander Zanden HB, Wunder MB, Hobson KA, Van Wilgenburg SL, Wassenaar LI, Welker JM, Bowen GJ. Space-time tradeoffs in the development of precipitation-based isoscape models to determining migratory origin. 2015. Journal of Avian Biology; 10.1111/jav.00656 [DOI] [Google Scholar]
- 66.IAEA Newscenter 2018. Available from https://www.iaea.org/newscenter/pressreleases/iaea-project-maps-groundwater-in-africas-sahel-region-shows-significant-reserves
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
75% Kernel density estimates are shown in red. a) Example of best geographical overlap. b) Example of least overlap.
(TIFF)
Data Availability Statement
All data files are available from the dryad digital repository database (doi:10.5061/dryad.72q5723 Data files: Seifert_et_al_Data_Barnswallows).