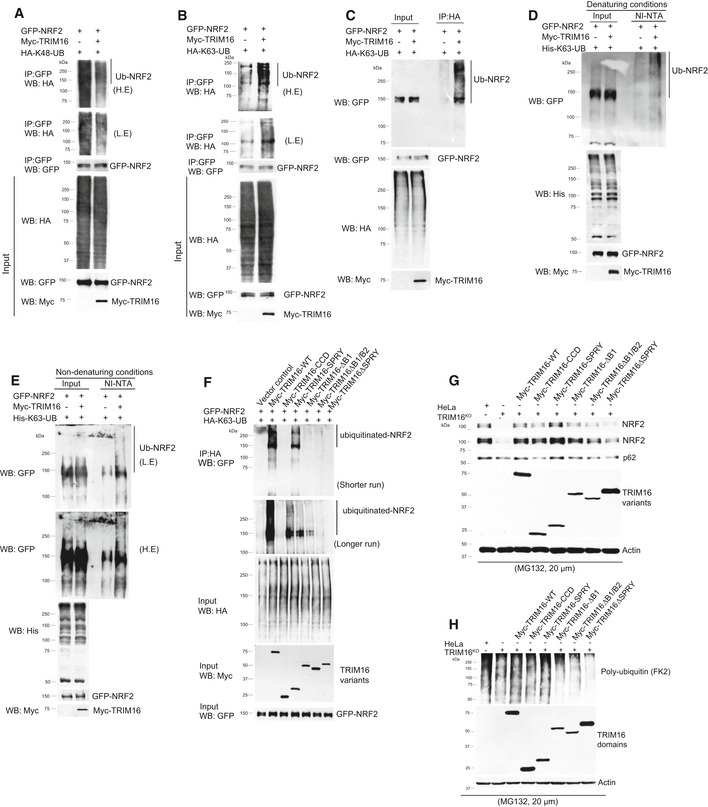
Figure 4. TRIM16 regulates ubiquitination of NRF2.
-
A–CAnalysis of NRF2 ubiquitination in absence and presence of TRIM16 by co‐IP assays using transiently transfected plasmid constructs as indicated. Two different variants of ubiquitin protein are used, one that can only be ubiquitinated at lysine 48 residue (HA‐K48‐UB) and other that can be only ubiquitinated at lysine 63 residue (HA‐K63‐UB). All other lysine residues are mutated. L.E, low exposure; H.E, high exposure.
-
D, EAnalysis of NRF2 ubiquitination in absence and presence of TRIM16 by Ni‐NTA pull‐down assays using transiently transfected plasmid constructs as indicated. His‐tagged ubiquitin which is mutated at all lysines except 63 position is used in these assays.
-
FAnalysis of NRF2 ubiquitination in absence and presence of TRIM16 deletion variants by co‐IP assays using transiently transfected plasmid constructs as indicated.
-
GWestern blot analysis of lysates from HeLa, TRIM16KO cells, and TRIM16KO cells complemented with TRIM16 deletion constructs where cells were treated with MG132 (20 μM, 2 h) and the blot is probed with indicated antibodies.
-
HWestern blot analysis of lysates from HeLa, TRIM16KO cells, and TRIM16KO cells complemented with TRIM16 deletion constructs where cells were treated with MG132 (20 μM, 2 h) and the blot is probed with indicated antibodies.
Source data are available online for this figure.
