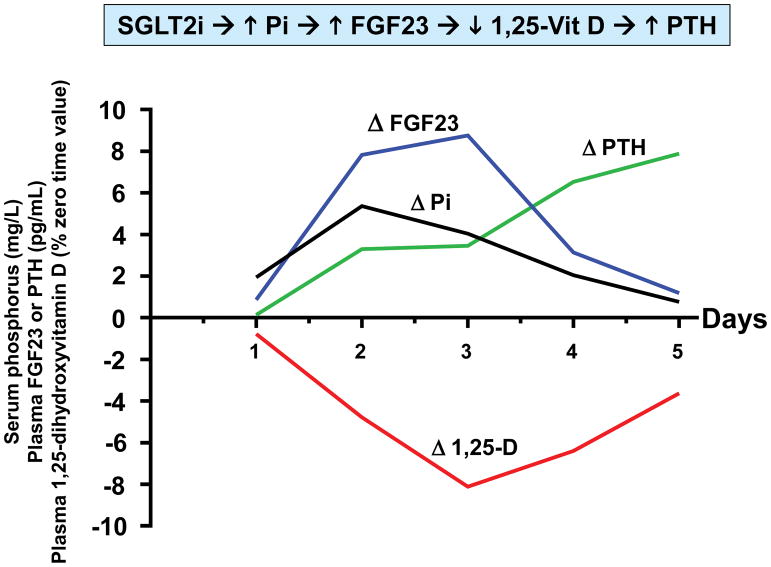
Figure 1. Time course of pharmacodynamic responses to canagliflozin.
Healthy volunteers (N=25) were admitted to the NIH Clinical Center to participate in a randomized crossover trial 5. Research subjects participated in two admissions during which they received either placebo or canagliflozin (300 mg/d) for five days. Each research subject was randomized between placebo or canagliflozin for the first admission, and then crossed over to the other treatment for the second admission. Four blood samples were obtained on each day (8 AM, 10 AM, 12 noon, and 8 PM). The details of the study design are described in Blau et al. 5 We calculated averages of the timed blood samples on each of the five days of the study. This figure presents drug-induced changes of those averages (i.e., placebo-subtracted values) for each day. In order to display all four parameters on the same axes, we expressed the data in the following units: serum phosphorus (mg/L); plasma FGF23 (pg/mL); plasma PTH (pg/mL); and 1,25-dihydroxyvitamin D (% of time zero value).