Figure 2. Working Model of the Pathway of Ubiquitination-Dependent BCR-Mediated Antigen Processing and Presentation.
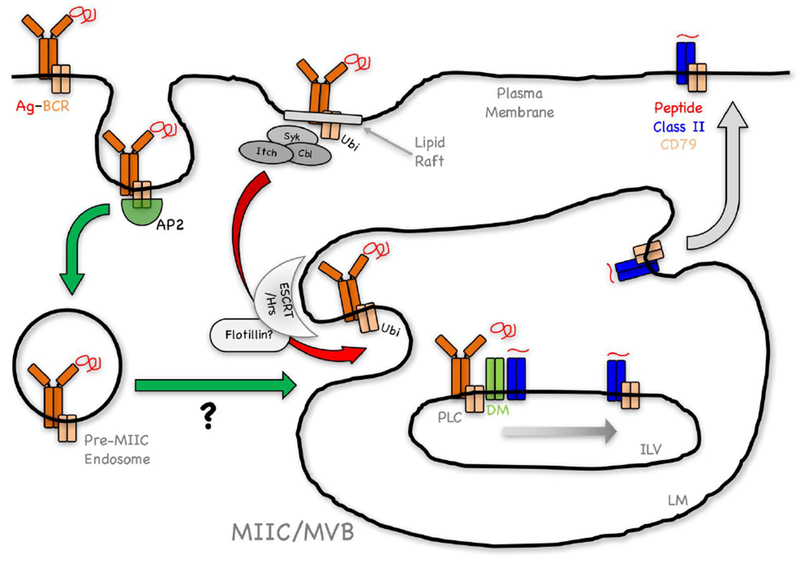
Antigen binding drives two concurrent events; signaling and Ag-BCR internalization. A fraction of Ag-BCR complexes not involved in signaling are internalized via an AP-2/CCP-dependent pathway (green arrow), mediated by the membrane proximal ITAM YxxØ motif of CD79B (see text). The ultimate intracellular fate of these complexes (delayed delivery to MIIC?) is currently unclear. A second population of Ag-BCR complexes within lipid rafts undergoes signaling-dependent ubiquitination (Ag-BCR-ubi) by either c-Cbl, Cbl-b and/or Itch (Figure 1, see text). Endocytosis of these Ag-BCR-ubi complexes via a poorly defined mechanism that may not be CCP (see text), results in their rapid delivery to the limiting membrane (LM) of multi-vesicular body (MVB)-like MIIC (red arrow). Recognition of these Ag-BCR-ubi complexes by the ESCRT/Hrs complex (possibly via a flotillin-dependent mechanism, see text) results in Ag-BCR de-ubiquitination and delivery to nascent intra-lumenal vesicles (ILV). Within ILV, Ag-BCR complexes associate with M1 paired MHC class II molecules (see text), associated with the class II chaperone HLA-DM/H-2M (DM). Within this MHC class II peptide loading complex (PLC), DM catalyzes Ag release from the BCR and the binding of antigen-derived peptides to the class II molecule. The PLC may also facilitate the transfer of the BCR CD79 signaling module to these nascent peptide-class II complexes. The resulting ILV peptide-MHC class II-CD79 complexes are then delivered to the MIIC LM by “back-fusion” and then to the cell surface for recognition by CD4 T cells.
