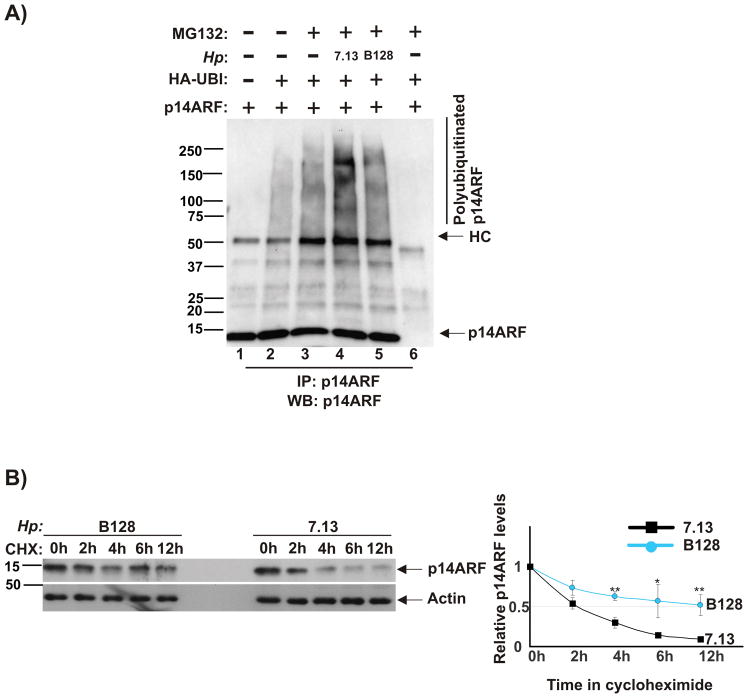
Figure 2. H. pylori strain 7.13 induces ubiquitination and degradation of p14ARF protein.
(A) AGS cells were co-transfected with plasmids expressing p14ARF and ubiquitin for 24 hours, co-cultured with strains 7.13 or B128 for 90 minutes and then incubated with proteasome inhibitor MG132 (20 μM) for 7 hours. Lysates were analyzed for polyubiquitinated p14ARF protein after its immunoprecipitation (IP) with p14ARF antibody. Gel loading was normal normalized to ARF protein. As a negative control for IP, normal rabbit IgG was used (lane 6). (B) Stability of p14ARF protein was analyzed in AGS cells transfected with p14ARF using the cycloheximide chase method (see the Material and Method section). Left panel: Expression of p14ARF protein was assessed by Western blotting. Right panel: Protein levels of p14ARF were quantified by densitometry and normalized to actin. ARF protein was quantitated by densitometry (p*<.05; p**<.01). Data are shown as mean ± SEM (n=2).