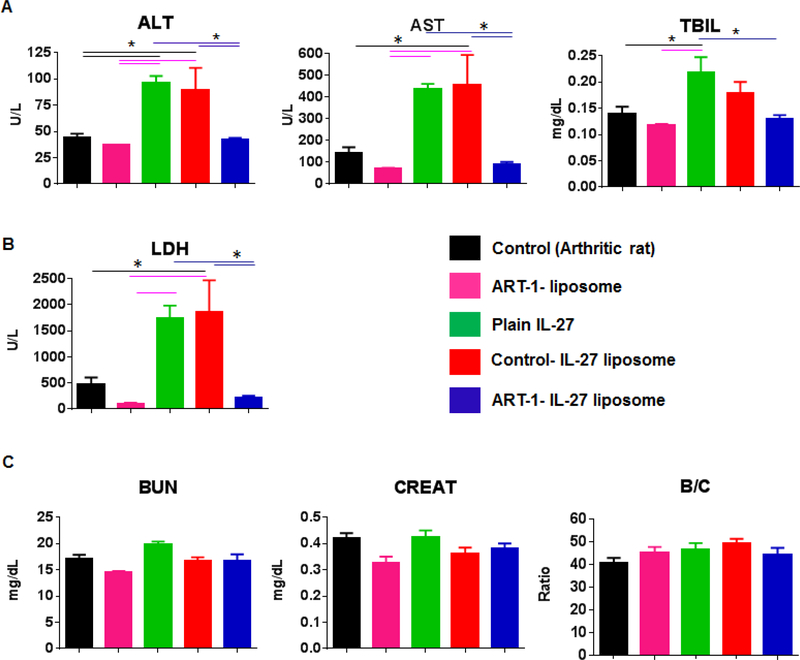
Figure 5:
Serum chemistry analysis to assess the systemic exposure profiles of various treatment modalities. Blood samples were collected from rats (n= 4–5 per group) subjected to different treatment regimen (shown in Fig 4) on d 25 after Mtb immunization and serum was separated for testing. Serum levels of (A) aspartate aminotransferase (AST), alanine transaminase (ALT), and total bilirubin (TBIL) indicative of hepatobiliary effects; (B) lactate dehydrogenase (LDH) indicative of acute/chronic tissue damage; and (C) blood urea nitrogen (BUN), creatinine (CREAT), and BUN/Creatinine (B/C) ratio indicative of renal effects were measured. (*, p<0.05)