Abstract
Apicomplexan parasites are responsible for a number of important human pathologies. Obviously, as Eukaryotes they share a number of cellular features and pathways with their respective host cells. One of them is autophagy, a process involved in the degradation of the cell's own components. These intracellular parasites nonetheless seem to present a number of original features compared to their very evolutionarily distant host cells. In mammals and other metazoans, autophagy has been identified as an important contributor to the defence against microbial pathogens. Thus, host autophagy also likely plays a key role in the control of apicomplexan parasites, although its potential manipulation and subversion by intracellular parasites creates a complex interplay in the regulation of host and parasite autophagy. In this mini-review, we summarise current knowledge on autophagy in both parasites and their host cells, in the context of infection by three Apicomplexa: Plasmodium, Toxoplasma, and Theileria.
Keywords: Autophagy, Plasmodium, Toxoplasma, Theileria, Cell signalling, Host cell
Autophagy in Apicomplexa
The core machinery for autophagy is evolutionarily conserved in most of the eukaryotic phyla, however Plasmodium, Toxoplasma and Theileria possess a reduced repertoire of recognizable autophagy-related proteins. Except in Toxoplasma, they noticeably lack clear orthologues of the initiating kinase ATG1/ULK1/2, and all lack proteins involved the nucleation of autophagosomes [Table 1]. Apicomplexan parasites also lack the equivalent of mammalian lysosomes, so they rather resemble fungi and plants by degrading autophagosome cargo in vacuoles with a proteolytic function. For example, in Plasmodium-infected red blood cells, autophagosomes fuse with the digestive food vacuole that is better known for degrading haemoglobin that the parasite imports from the erythrocyte cytosol [1]. Plasmodium sporozoites and merozoites are the developmental stages invasive for hepatocytes and erythrocytes, respectively, but they do not possess a food vacuole. However, it has been proposed that post-invasion of hepatocytes, Plasmodium berghei ATG8-decorated micronemes (an invasion-related organelle) are expelled from the parasite and degraded by enzymes present in the parasitophorous vacuole (PV) lumen [2]. Proposing the PV as a degradative compartment is an interesting concept, as invasive Toxoplasma tachyzoites leave behind a residual body of unused material after their division by endodyogeny, which vanishes quite rapidly as parasites develop in the vacuole. Therefore, the PV might be an important interface between the parasite and its host cell for nutrient acquisition, where import of autophagosome-recycled parasite material from the lumen back into the parasite might be facilitated. One should point out that post-invasion of leukocytes or erythrocytes, Theileria parasites reside only very transiently within a PV that is rapidly degraded, leaving the parasites exposed to the host cell cytosol [3]. If secretory autophagy occurs, then lysosomes in the host cell cytosol could be the digestive compartment for Theileria-derived autophagosome cargo.
Table 1.
Putative homologues of Saccharomyces cerevisiae Atg proteins in Theileria, Plasmodium and Toxoplasma. Yeast sequences were used as a BLAST query in PiroplasmaDB.org, PalsmoDB.org, and ToxoDB.org, respectively. Evidence of in vivo phosphorylation was also searched for in the PlasmoDB.org and ToxoDB.org databases. nf: no homologue found,*: distant homologue (e-value >10e–5).
| Functional group | Yeast protein | T. annulata orthologue | P. falciparum orthologue | Phosphorylation | T. gondii orthologue | Phosphorylation | Features and possible function |
|---|---|---|---|---|---|---|---|
| Atg1 complex | Atg1 | multiple possible hits | multiple possible hits | TGME49_316150* | No | Ser/Thr protein kinase; regulated by the TOR complex; recruitment of Atg proteins to the phagophore assembly site | |
| Atg13 | nf | nf | nf | Regulatory subunit through phosphorylation by TORC1 or PKA | |||
| Atg17 | nf | nf | nf | Scaffold protein | |||
| Atg29 | nf | nf | nf | Ternary complex with Atg17 and Atg31; not found in mammals | |||
| Atg31 | nf | nf | nf | Ternary complex with Atg17 and Atg29; not found in mammals | |||
| Atg11 | nf | PF3D7_0216700* | Yes | nf | Scaffold protein for phagophore assembly in selective autophagy; not found in mammals | ||
| Atg9 and its cycling system | Atg2 | nf | nf | TGME49_304630 | Yes | Interacts with Atg18 | |
| Atg9 | nf | nf | TGME49_260640 | Yes | Transmembrane protein, possible membrane carrier for phagophore formation | ||
| Atg18/A tg21 | TA03100 | PF3D7_1012900 | Yes | TGME49_288600 TGME49_220160 | Yes | PtdIns3P-binding protein; potentially involved in driving membrane elongation | |
| PtdIns3K complex | Vps34 | TA20360 | PF3D7_0515300 | Yes | TGME49_215700 | Yes | PtdIns 3-kinase |
| Vps15 | TA04815 | PF3D7_0823000 | Yes | TGME49_310190 | Yes | Ser/Thr protein kinase | |
| Vps30/Atg6 | nf | nf | TGME49_221360 | Yes | Component of the PtdIns3K complex | ||
| Atg14 | nf | nf | nf | Component of the PtdIns3K complex | |||
| Atg8 and Atg12 Ubiquitin-like conjugation systems | Atg8 | TA03605 | PF3D7_1019900 | Yes | TGME49_254120 | No | Ubiquitin-like; conjugated to PE at the autophagosome membrane; involved in autophagosome cargo recognition and possibly in membrane elongation |
| Atg7 | TA06610 | PF3D7_1126100 | No | TGME49_229690 | Yes | E1-like enzyme | |
| Atg3 | TA03605 | PF3D7_0905700 | Yes | TGME49_236110 | Yes | E2-like enzyme | |
| Atg4 | TA13550 | PF3D7_1417300 | Yes | TGME49_206450* | Yes | Cysteine protease; deconjugating enzyme for Atg8 | |
| Atg12 | TA11895* | PF3D7_1470000 | No | TGME49_321300 | Yes | Ubiquitin-like | |
| Atg10 | nf | nf | nf | E2-like enzyme | |||
| Atg16 | nf | nf | TGME49_200280* | Yes | Interacts with Atg5 and Atg12 | ||
| Atg5 | TA04165 | PF3D7_1430400* | No | TGME49_230860* | Yes | Conjugated by Atg12 | |
| Other | Rab7 | TA17640 | PF3D7_0903200 | Yes | TGME49_248880 | Yes | Late endodomes/lysosomes marker; involved in the final maturation of late autophagic vacuoles in mammals |
Autophagy in Plasmodium parasites
A better understanding of autophagy regulation in malaria-causing Plasmodium species has taken on renewed urgency due to the recent description of artemisinin-resistance mutations occurring in Plasmodium falciparum Atg18 (PfAtg18) [4]. In addition, previously, resistance to another anti-malaria drug (chloroquine) was associated with alterations in PfATG8 distribution [5]. Although PfATG18 has not yet been characterised, studies on PfATG8 are well documented (14 papers in PubMed). Particularly, a surprisingly common observation was the localisation of PfATG8 on a non-photosynthetic plastid, present in most apicomplexan parasites, called the apicoplast [6], [7]. This led to the proposition that PfATG8 has a non-canonical function in apicoplast biogenesis [1] or, since apicoplasts also bind Phosphatidylinositol 3-phosphate (PI3P) produced by Vps34, its membrane might be the site of phagophore formation [2]. Once formed, the maturation of autophagosomes is associated with them becoming decorated with PfRab7, and then fusing with the food vacuole for degradation of their cargo [1]. PI3P binds to FYVE-domains [8] and the single parasite FYVE domain-containing protein also locates to the food vacuole [9], where it might participate in fusion of the autophagosome with the food vacuole membrane. The function of autophagy in Plasmodium blood stages is largely unexplored, but one proteomic study suggested that PfATG8 could be involved in parasite ribophagy and piecemeal microautophagy of the nucleus [10].
In the absence of a recognizable ATG1 orthologue (see Table 1, [11]) it's intriguing as to how malaria parasites regulate the initiation of autophagy and one can only hypothesize that another unidentified parasite kinase activity might play an ATG1-like role. Clearly, little is known and one possibility is that post-translational modifications of ATG proteins play a dominant role in regulating autophagy. In Table 1 we have indicated the phosphorylation status collated from PlasmoDB (http://plasmodb.org) of the different PfATG proteins and most are phosphorylated at more than one site. One can see that only PfATG5, PfATG7 and PfATG12 are not phosphorylated in infected red blood cells. cAMP-dependent protein kinase A (PKA) likely plays an important regulatory role, as PfATG4 (T625), PfATG8 (T83), PfATG11 (S243, S465), PfVps34 (T47, S90, S1036, S1362), PfVps15 (S250) and PfRab7 (S72) are all phosphorylated in vivo at typical PKA sites. Other phospho-sites and the two in PfATG18 (S42, S375) are not typical of PKA suggesting that additional parasite kinases must be responsible. Clearly then, kinases and phosphatases are likely key players in the regulation of parasite autophagy.
Autophagy in Plasmodium-infected host cells
Although autophagy is well studied as reticulocytes develop into normocytes, a process during which organelles including the nucleus are eliminated during erythropoiesis [12], little is known about host cell autophagy in Plasmodium-infected mature erythrocytes. However, in P. berghei-infected hepatocytes the PV membrane (PVM) is decorated with LC3 (“microtubule-associated protein 1A/1B-light chain 3”, the mammalian orthologue of ATG8), ubiquitin, SQSTM1/p62 and lysosomes in a process resembling selective autophagy [13]. As P. berghei development is dampened in host hepatocytes deficient in autophagy, it gave rise to the proposition that host cell autophagy was occurring at the PVM to supply the parasite with nutrients necessary for optimal growth [13], [14]. Moreover, in human hepatocytes infected with Plasmodium vivax, interferon-gamma (IFN-γ) stimulation also enhances LC3 and lysosome recruitment to the PVM [15]. However, this IFN-γ mediated induction of autophagy seemed detrimental to liver-stage P. vivax infection, in contrast to the role described promoting P. berghei development [13], [14]. Moreover, IFN-γ mediated induction of autophagy appeared non-canonical, as it did not involve activation of the mammalian ATG1 orthologue ULK1. Thus, during liver stage infection the parasite provokes hepatocyte autophagy to help it grow, while the host appears to respond to infection by IFN-γ stimulated autophagy to eliminate the parasite.
Autophagy in Toxoplasma parasites
Toxoplasma tachyzoites (rapidly dividing forms of the parasite) can generate autophagosome-like structures upon experiencing stress, for instance in the case of nutrient deprivation, both for extracellular [16] and intracellular [17] parasites. Electron microscopy imaging revealed the presence of cytoplasm-containing double-membrane autophagosomes and potential autophagolysosomes in starved parasites [16], [17]. In Toxoplasma, GFP-fused TgATG8 was used to detect and quantify autophagic vesicles [16]: upon starvation, the protein re-localizes from the cytosol to punctate structures that by immuno-electron microscopy resemble autophagosomes. Noticeably, prolonged starvation triggers significant parasite mortality and leads to the disruption of the mitochondrial network in Toxoplasma tachyzoites. The fact that this can be prevented by the use of a chemical inhibitor of autophagy suggests that autophagic cell death could be involved [17]. Functional investigation of a Toxoplasma ATG9 homologue (a protein potentially important for the early steps of autophagosome formation), revealed a possible role for canonical autophagy in the parasites for surviving stress conditions, either as extracellular parasites or within host immune cells [18]. Altogether, these data suggest that canonical autophagy could be part of an integrated stress response pathway in Toxoplasma, although there is no clear demonstration of a fully functional parasite catabolic autophagy.
Surprisingly, under normal intracellular growth conditions, TgATG8 localizes to the membrane of the apicoplast [19], [20], as described above for Plasmodium. This peculiar organelle harbours essential metabolic pathways, and cell lines deficient for TgATG8 [21] and related proteins TgATG3 [16] and TgATG4 [19] (that regulates TgATG8 membrane association), have converging phenotypes showing loss of both the apicoplast and parasite viability. This illustrates that part of the autophagy machinery is used for associating TgATG8 to the apicoplast, where it plays a vital role in organelle inheritance during cell division [21]. This important function appears clearly distinct from canonical autophagy and highlights that apicomplexan parasites may have subverted at least part of the machinery for performing a specialized non-canonical function [22].
Host cell autophagy for the control of Toxoplasma
Toxoplasma gondii is an obligate intracellular parasite that invades a wide range of vertebrate host cells. In these, autophagy has been identified as an important contributor to the defense against microbial pathogens (including viruses, bacteria and parasitic protists) [23]. Not only does autophagy allow the selective delivery of intracellular pathogens to the lysosomes for their degradation (a process called xenophagy), but microbial antigens generated through this process can also be used for the activation of innate and adaptive immunity. The recruitment of LC3 to single-membrane phagosomes surrounding intracellular bacteria has also been described recently and termed LC3-associated phagocytosis (LAP) [15], [24]. This suggests that observing LC3 around pathogens can no longer by itself be taken as an evidence for the presence of autophagosomes, and that some other unconventional compartments involving autophagic markers might be involved in their elimination.
In mammalian cells, efficient control of T. gondii infections is achieved by IFN-γ, a cytokine that triggers the activation of a diverse array of effector pathways, including NO production, nutrient starvation, and the induction of immunity-related GTPase (IRG proteins - rodent-specific GTPases), or GBP (guanylate-binding proteins) proteins that damage the PV membrane [25]. In recent years, a number of reports have suggested a role for the host cell autophagy machinery in the control of Toxoplasma tachyzoites. In the mouse model, IRGs promote the elimination of Toxoplasma by associating with the PV in an IFN-γ-dependent way, leading to the disruption of its membrane, and exposure of the parasite to the host cytoplasm and its eventual elimination [26]. Early reports were already describing the recruitment of both IRGs and autophagy protein LC3 to the vicinity of the PV [27] [26]. Since then, numerous studies have convincingly shown that several members of the host autophagy machinery are important for IRG recruitment at the PV membrane and subsequent parasite clearance. Noticeably, ATG3, ATG5, ATG7, ATG12 and ATG16L1, which are all involved in the mechanism of LC3 conjugation to membranes, are also important for IFN-γ-inducible IRG localization to the PV membrane [28]. LC3 is thus likely the key player in this, and it was indeed confirmed that this protein, and also its homologues GABARAPL1 and GABARAPL2, are needed for targeting of the IRGs to the PV [29]. Once associated with the PV membrane, LC3 could ‘tag’ it for targeting by IRGs, and maybe even act as a scaffold to recruit the GTPases for subsequent parasite elimination [30]. However, to date there is no proof of a direct interaction between LC3 and IRGs. Nevertheless, it seems clear that this mechanism is independent of canonical autophagy, as interfering with the function of ULK1, ULK2, ATG9 and ATG14 did not impact on LC3 and IRG recruitment to the PVM, or subsequent elimination of parasites [30], [31].
Noticeably, human cells do not express IRGs, but GBPs, which can also be recruited to the PVM through the LC3 conjugation system [31]. However, GBPs are not essential for IFN-γ-dependent parasite elimination and a recent study in human cells has shown core autophagy proteins involved in LC3 conjugation are important in the control of parasite growth in a different way: in this case, they seem to be required for a process that results in wrapping the PV in multiple host membranes to limit parasite growth [32].
CD40 [a member of the tumor necrosis factor receptor superfamily] signaling has also been shown to trigger autophagic elimination of T. gondii independently of IFN-γ and IRGs [33], [34], [35], [36]. This anti-parasitic activity also depends on proteins of the autophagy machinery, and the sequestration and degradation of intracellular tachyzoites occurs possibly through classical autophagy, although this remains to be more firmly established.
Finally, in parallel with the implication of host autophagy in the control of intracellular microbes, there is also a growing list of pathogens that seem to be able to antagonize the host autophagy machinery, or even exploit it to enhance their replication. A couple of reports suggest Toxoplasma might be able to do so in the case of permissive and non-immune host cells. For example, T. gondii can activate the epidermal growth factor receptor (EGFR)/Akt pathway to prevent the host autophagy machinery from targeting the parasite for lysosomal degradation [37]. Moreover, T. gondii is able to induce a significant recruitment of the autophagy machinery in HeLa cells or primary fibroblasts, where genetic inactivation of the autophagic function limits parasite growth, suggesting a beneficial role for host cell autophagy in the development of the parasites [38].
In conclusion, a clearance function of the autophagy machinery enhances pathogen killing in host cells that have been activated for anti-parasitic function, while in permissive host cells tachyzoites may co-opt the autophagy machinery for their own benefit.
Modulation of autophagy by Theileria: at the crossroads of infection, immunity and tumorigenesis
Theileria belongs to the same Apicomplexa phylum as Plasmodium and Toxoplasma, but it has developed distinct mechanisms in its parasitic life cycle that make these parasites unique among the known Apicomplexa [39]. Unlike Toxoplasma and Plasmodium, Theileria parasites do not reside within a PV within host leukocytes, so are exposed to the host cell's autophagy machinery [3], [40]. Nevertheless, the host autophagy machinery does not appear to react to the presence of the parasite that surprisingly persists in the cell in an almost 1:1 leukocyte:parasite ratio. Thus, even when Theileria is somewhat exposed due to the absence of the PV, infected host cell autophagy is not induced [41]. How these parasites are able to avoid cell autophagy to survive in infected leukocytes is still unclear. Recent work suggests that Theileria might block some of the autophagy pathways in the infected host cell, because when Theileria is killed with an anti-parasite drug, dead parasites are immediately engulfed by LC3-positive structures (Latré de Laté and Pineda, unpublished).
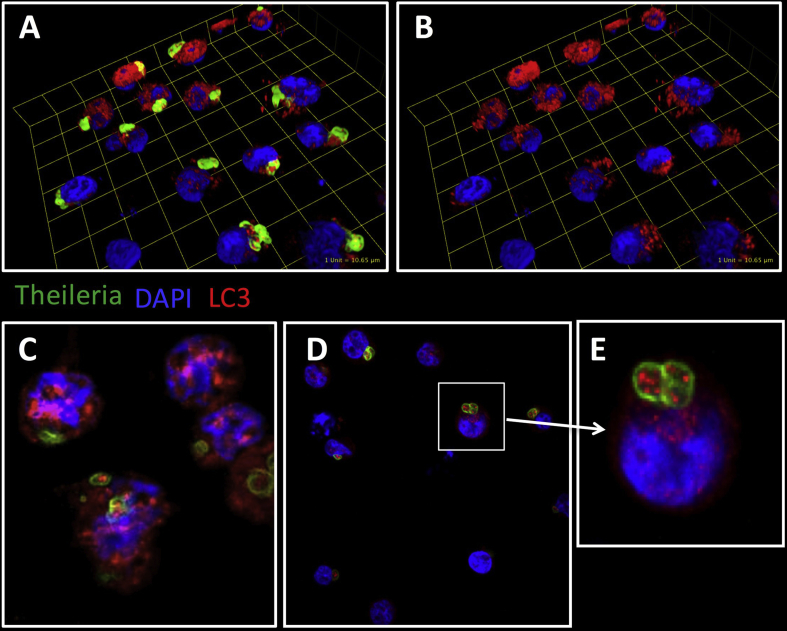
Another extraordinary difference to Plasmodium and Toxoplasma is that Theileria transforms its host cells into an immortalized, highly proliferative and disseminating phenotype, properties shared by many cancer types [39]. This is presumably achieved through mechanisms triggered by the parasite to manipulate leukocyte signal transduction pathways that are also relevant for tumorigenesis. Indeed, in infected macrophages, Theileria annulata maintains a Hypoxia Inducible Factor (HIF)-1α-driven transcriptional programme typical of Warburg glycolysis [42], a hallmark of cancer cells. Thus, Theileria infection represents a valuable model for studying cancer and lymphoproliferation. In this regard, defects in autophagy have been associated with increased tumorigenesis in some cancers [43], so perhaps Theileria's regulation of host autophagy is the mechanism underlying parasite survival and leukocyte transformation, events that would not be independent, but closely intertwined, although the exact mechanisms are still unknown. Theileria induces the oncomiR miR-155, via the c-Jun transcription factor and AP-1 activity [44]. In turn, miR-155 modulates cell autophagy, promoting it to clear intracellular mycobacteria [45], or suppressing it during osteoarthritis and cancer [46], [47]. Furthermore, cAMP-PKA signalling is upregulated by Theileria infection [48] and it is well established in different cell types that cAMP-PKA signalling negatively regulates autophagy (see accompanying review and [49], [50], [51], [52]). cAMP-PKA-mediated phosphorylation of LC3 blocks autophagy. This is mimicked when cAMP production is stimulated by forskolin (an adenylate cyclase activator) and prevented by PKA inhibitor H89. Under conditions of costimulation, LC3 phosphorylation is diminished, leading to autophagy [49]. As augmented cAMP-PKA signaling is characteristic of Theileria-infected leukocytes, increased LC3 phosphorylation can be observed and this likely contributes to infection, perhaps inducing blockade in the host's autophagy response. It is also well known that Theileria infection induces increased JNK kinase activities [53], [54]. Recently it has been shown that loss of JNK2 leads to accumulation of smARF and lysosomal degradation of the adaptor p62 (sequestosome-1, SQSTM1) [55] implying that constitutive induction of JNK2 following Theileria-infection could also contribute to the observed stable levels of p62 that reflect a blockade of the host leukocyte's autophagic response. Consistent with this notion, one can observe a decrease in p62 expression only when infected leukocytes are treated with a pro-oxidant anti-parasite drug (Latré de Laté and Pineda, unpublished). Interestingly, when expression of LC3 and phosphorylated LC3 (p-LC3) is evaluated in infected macrophages, a positive staining is also observed within the parasites [Fig. 1]. Whether this is a result of parasite uptake of host LC3 is still unclear and requires further investigation, but it strongly suggests that host and/or parasite autophagy play a relevant role in parasite survival within the infected cell, and perhaps by sequestering host LC3 within itself, Theileria inhibits autophagy.
Fig. 1.
LC3 can be detected around and inside Theileria annulata-infected macrophages. Visualisation of intracellular parasites (green; 1C12 monoclonal antibody) [56] and LC3 (red; rabbit polyclonal anti-LC3α/β antibody that recognises endogenous host LC3 [Santa Cruz Biotechnology, cat. No sc-292354] and Alexa Fluor 594-conjugated anti-rabbit Ig antibody) in T. annulata-infected macrophages. DAPI was used to counter-stain macrophage nuclei. The 3D-representations of spatial co-localizations of parasite and LC3 (A), as revealed by the removal of parasite staining (B). Confocal microscopy (C, D) also shows LC3 staining within the parasite, magnified in (E). Images were acquired by Spinning Disk Confocal Microscopy and analysed by ImageJ and Volocity software.
In conclusion, to survive, it appears that while exposed within the cytoplasm of host leukcoytes Theileria parasites might modulate the induction of autophagy by targeting at least three different steps in the autophagy induction process: cAMP-PKA-mediated phosphorylation of LC3, JNK2-mediated blockade in p62 degradation, and LC3 sequestration. Understanding how Theileria regulates host autophagy will provide novel pathways to improve our current knowledge of autophagy both in infection and in cancer.
Conflicts of interest
The authors have no conflict of interest to declare regarding this manuscript.
Acknowledgments
PLdL was supported by a ParaFrap post-doctoral fellowship. MAP is an Arthritis Research UK Career Development Fellow. SB and GL acknowledge support from ANR-11-LABX-0024 and the CNRS; SB acknowledges grant ANR-13-JSV3-0003 and GL acknowledges INSERM support.
Footnotes
Peer review under responsibility of Chang Gung University.
Contributor Information
Margaret Harnett, Email: Margaret.Harnett@glasgow.ac.uk.
Gordon Langsley, Email: gordon.langsley@inserm.fr.
References
- 1.Tomlins A.M., Ben-Rached F., Williams R.A., Proto W.R., Coppens I., Ruch U. Plasmodium falciparum ATG8 implicated in both autophagy and apicoplast formation. Autophagy. 2013;9:1540–1552. doi: 10.4161/auto.25832. [DOI] [PubMed] [Google Scholar]
- 2.Voss C., Ehrenman K., Mlambo G., Mishra S., Kumar K.A., Sacci J.B., Jr. Overexpression of Plasmodium berghei ATG8 by liver forms leads to cumulative defects in organelle dynamics and to generation of noninfectious merozoites. MBio. 2016;7 doi: 10.1128/mBio.00682-16. e00682–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw M.K., Tilney L.G. The entry of Theileria parva merozoites into bovine erythrocytes occurs by a process similar to sporozoite invasion of lymphocytes. Parasitology. 1995;111(Pt 4):455–461. doi: 10.1017/s0031182000065951. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z., Cabrera M., Yang J., Yuan L., Gupta B., Liang X. Genome-wide association analysis identifies genetic loci associated with resistance to multiple antimalarials in Plasmodium falciparum from China-Myanmar border. Sci Rep. 2016;6:33891. doi: 10.1038/srep33891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaviria D., Paguio M.F., Turnbull L.B., Tan A., Siriwardana A., Ghosh D. A process similar to autophagy is associated with cytocidal chloroquine resistance in Plasmodium falciparum. PLoS One. 2013;8:e79059. doi: 10.1371/journal.pone.0079059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitamura K., Kishi-Itakura C., Tsuboi T., Sato S., Kita K., Ohta N. Autophagy-related Atg8 localizes to the apicoplast of the human malaria parasite Plasmodium falciparum. PLoS One. 2012;7:e42977. doi: 10.1371/journal.pone.0042977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eickel N., Kaiser G., Prado M., Burda P.C., Roelli M., Stanway R.R. Features of autophagic cell death in Plasmodium liver-stage parasites. Autophagy. 2013;9:568–580. doi: 10.4161/auto.23689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raiborg C., Schink K.O., Stenmark H. Class III phosphatidylinositol 3-kinase and its catalytic product PtdIns3P in regulation of endocytic membrane traffic. FEBS J. 2013;280:2730–2742. doi: 10.1111/febs.12116. [DOI] [PubMed] [Google Scholar]
- 9.McIntosh M.T., Vaid A., Hosgood H.D., Vijay J., Bhattacharya A., Sahani M.H. Traffic to the malaria parasite food vacuole: a novel pathway involving a phosphatidylinositol 3-phosphate-binding protein. J Biol Chem. 2007;282:11499–11508. doi: 10.1074/jbc.M610974200. [DOI] [PubMed] [Google Scholar]
- 10.Cervantes S., Bunnik E.M., Saraf A., Conner C.M., Escalante A., Sardiu M.E. The multifunctional autophagy pathway in the human malaria parasite, Plasmodium falciparum. Autophagy. 2014;10:80–92. doi: 10.4161/auto.26743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foldvari-Nagy L., Ari E., Csermely P., Korcsmaros T., Vellai T. Starvation-response may not involve Atg1-dependent autophagy induction in non-unikont parasites. Sci Rep. 2014;4:5829. doi: 10.1038/srep05829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fader C.M., Colombo M.I. Multivesicular bodies and autophagy in erythrocyte maturation. Autophagy. 2006;2:122–125. doi: 10.4161/auto.2.2.2350. [DOI] [PubMed] [Google Scholar]
- 13.Prado M., Eickel N., De Niz M., Heitmann A., Agop-Nersesian C., Wacker R. Long-term live imaging reveals cytosolic immune responses of host hepatocytes against Plasmodium infection and parasite escape mechanisms. Autophagy. 2015;11:1561–1579. doi: 10.1080/15548627.2015.1067361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thieleke-Matos C., Lopes da Silva M., Cabrita-Santos L., Portal M.D., Rodrigues I.P., Zuzarte-Luis V. Host cell autophagy contributes to Plasmodium liver development. Cell Microbiol. 2016;18:437–450. doi: 10.1111/cmi.12524. [DOI] [PubMed] [Google Scholar]
- 15.Boonhok R., Rachaphaew N., Duangmanee A., Chobson P., Pattaradilokrat S., Utaisincharoen P. LAP-like process as an immune mechanism downstream of IFN-gamma in control of the human malaria Plasmodium vivax liver stage. Proc Natl Acad Sci U S A. 2016;113:E3519–E3528. doi: 10.1073/pnas.1525606113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Besteiro S., Brooks C.F., Striepen B., Dubremetz J.-F. Autophagy protein Atg3 is essential for maintaining mitochondrial integrity and for normal intracellular development of Toxoplasma gondii tachyzoites. PLoS Pathog. 2011;7:e1002416. doi: 10.1371/journal.ppat.1002416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh D., Walton J.L., Roepe P.D., Sinai A.P. Autophagy is a cell death mechanism in Toxoplasma gondii. Cell Microbiol. 2012;14:589–607. doi: 10.1111/j.1462-5822.2011.01745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen H.M., El Hajj H., El Hajj R., Tawil N., Berry L., Lebrun M. Toxoplasma gondii autophagy-related protein ATG9 is crucial for the survival of parasites in their host. Cell Microbiol. 2017 doi: 10.1111/cmi.12712. in press. [DOI] [PubMed] [Google Scholar]
- 19.Kong-Hap M.A., Mouammine A., Daher W., Berry L., Lebrun M., Dubremetz J.-F. Regulation of ATG8 membrane association by ATG4 in the parasitic protist Toxoplasma gondii. Autophagy. 2013;9:1334–1348. doi: 10.4161/auto.25189. [DOI] [PubMed] [Google Scholar]
- 20.Lavine M.D., Arrizabalaga G. Analysis of monensin sensitivity in Toxoplasma gondii reveals autophagy as a mechanism for drug induced death. PLoS ONE. 2012;7:e42107. doi: 10.1371/journal.pone.0042107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lévêque M.F., Berry L., Cipriano M.J., Nguyen H.-M., Striepen B., Besteiro S. Autophagy-related protein ATG8 has a noncanonical function for apicoplast inheritance in Toxoplasma gondii. mBio. 2015;6 doi: 10.1128/mBio.01446-15. e01446–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lévêque M.F., Nguyen H.M., Besteiro S. Repurposing of conserved autophagy-related protein ATG8 in a divergent eukaryote. Commun Integr Biol. 2016;9:e1197447. doi: 10.1080/19420889.2016.1197447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomes Ligia C., Dikic I. Autophagy in antimicrobial immunity. Mol Cell. 2014;54:224–233. doi: 10.1016/j.molcel.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Lai S.-C., Devenish R.J. LC3-Associated phagocytosis (LAP): connections with host autophagy. Cells. 2012;1:396–408. doi: 10.3390/cells1030396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yarovinsky F. Innate immunity to Toxoplasma gondii infection. Nat Rev Immunol. 2014;14:109–121. doi: 10.1038/nri3598. [DOI] [PubMed] [Google Scholar]
- 26.Martens S., Parvanova I., Zerrahn J., Griffiths G., Schell G., Reichmann G. Disruption of Toxoplasma gondii parasitophorous vacuoles by the mouse p47-resistance GTPases. PLoS Pathog. 2005;1:e24. doi: 10.1371/journal.ppat.0010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ling Y.M., Shaw M.H., Ayala C., Coppens I., Taylor G.A., Ferguson D.J.P. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J Exp Med. 2006;203:2063–2071. doi: 10.1084/jem.20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Z., Fux B., Goodwin M., Dunay I.R., Strong D., Miller B.C. Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell Host Microbe. 2008;4:458–469. doi: 10.1016/j.chom.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park S., Choi J., Biering S.B., Dominici E., Williams L.E., Hwang S. Targeting by AutophaGy proteins (TAG): targeting of IFNG-inducible GTPases to membranes by the LC3 conjugation system of autophagy. Autophagy. 2016;12:1153–1167. doi: 10.1080/15548627.2016.1178447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi J., Biering S.B., Hwang S. Quo vadis? Interferon-inducible GTPases go to their target membranes via the LC3-conjugation system of autophagy. Small GTPases. 2016:1–9. doi: 10.1080/21541248.2016.1213090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohshima J., Lee Y., Sasai M., Saitoh T., Su Ma J., Kamiyama N. Role of mouse and human autophagy proteins in IFN-gamma-induced cell-autonomous responses against Toxoplasma gondii. J Immunol. 2014;192:3328–3335. doi: 10.4049/jimmunol.1302822. [DOI] [PubMed] [Google Scholar]
- 32.Selleck E.M., Orchard R.C., Lassen K.G., Beatty W.L., Xavier R.J., Levine B. A noncanonical autophagy Pathway restricts Toxoplasma gondii growth in a strain-specific manner in IFN-γ-activated human cells. mBio. 2015;6 doi: 10.1128/mBio.01157-15. e01157–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andrade R.M., Wessendarp M., Gubbels M.-J., Striepen B., Subauste C.S. CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J Clin Invest. 2006;116:2366–2377. doi: 10.1172/JCI28796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu E., Van Grol J., Subauste C.S. Atg5 but not Atg7 in dendritic cells enhances IL-2 and IFN-γ production by Toxoplasma gondii-reactive CD4+ T cells. Microbes Infect. 2015;17:275–284. doi: 10.1016/j.micinf.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu E., Lopez Corcino Y., Portillo J.-A.C., Miao Y., Subauste C.S. Identification of signaling pathways by which CD40 stimulates autophagy and antimicrobial activity against Toxoplasma gondii in macrophages. Infect Immun. 2016;84:2616–2626. doi: 10.1128/IAI.00101-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Grol J., Muniz-Feliciano L., Portillo J.A.C., Bonilha V.L., Subauste C.S. CD40 induces anti-Toxoplasma gondii activity in nonhematopoietic cells dependent on autophagy proteins. Infect Immun. 2013;81:2002–2011. doi: 10.1128/IAI.01145-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muniz-Feliciano L., Van Grol J., Portillo J.-A.C., Liew L., Liu B., Carlin C.R. Toxoplasma gondii-induced activation of EGFR prevents autophagy protein-mediated killing of the parasite. PLoS Pathog. 2013;9:e1003809. doi: 10.1371/journal.ppat.1003809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y., Weiss L.M., Orlofsky A. Host cell autophagy is induced by Toxoplasma gondii and contributes to parasite growth. J Biol Chem. 2008;284:1694–1701. doi: 10.1074/jbc.M807890200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dobbelaere D., Heussler V. Transformation of leukocytes by Theileria parva and T. annulata. Annu Rev Microbiol. 1999;53:1–42. doi: 10.1146/annurev.micro.53.1.1. [DOI] [PubMed] [Google Scholar]
- 40.Shaw M.K. Cell invasion by Theileria sporozoites. Trends Parasitol. 2003;19:2–6. doi: 10.1016/s1471-4922(02)00015-6. [DOI] [PubMed] [Google Scholar]
- 41.Duszenko M., Ginger M.L., Brennand A., Gualdron-Lopez M., Colombo M.I., Coombs G.H. Autophagy in protists. Autophagy. 2011;7:127–158. doi: 10.4161/auto.7.2.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Metheni M., Echebli N., Chaussepied M., Ransy C., Chereau C., Jensen K. The level of H(2)O(2) type oxidative stress regulates virulence of Theileria-transformed leukocytes. Cell Microbiol. 2014;16:269–279. doi: 10.1111/cmi.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maes H., Rubio N., Garg A.D., Agostinis P. Autophagy: shaping the tumor microenvironment and therapeutic response. Trends Mol Med. 2013;19:428–446. doi: 10.1016/j.molmed.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Marsolier J., Pineau S., Medjkane S., Perichon M., Yin Q., Flemington E. OncomiR addiction is generated by a miR-155 feedback loop in Theileria-transformed leukocytes. PLoS Pathog. 2013;9:e1003222. doi: 10.1371/journal.ppat.1003222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J., Yang K., Zhou L., Wu M., Wu Y., Zhu M. MicroRNA-155 promotes autophagy to eliminate intracellular mycobacteria by targeting Rheb. PLoS Pathog. 2013;9:e1003697. doi: 10.1371/journal.ppat.1003697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D'Adamo S., Alvarez-Garcia O., Muramatsu Y., Flamigni F., Lotz M.K. MicroRNA-155 suppresses autophagy in chondrocytes by modulating expression of autophagy proteins. Osteoarthr Cartil. 2016;24:1082–1091. doi: 10.1016/j.joca.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen L., Jiang K., Jiang H., Wei P. miR-155 mediates drug resistance in osteosarcoma cells via inducing autophagy. Exp Ther Med. 2014;8:527–532. doi: 10.3892/etm.2014.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guergnon J., Dessauge F., Traincard F., Cayla X., Rebollo A., Bost P.E. A PKA survival pathway inhibited by DPT-PKI, a new specific cell permeable PKA inhibitor, is induced by T. annulata in parasitized B-lymphocytes. Apoptosis. 2006;11:1263–1273. doi: 10.1007/s10495-006-7702-6. [DOI] [PubMed] [Google Scholar]
- 49.Cherra S.J., 3rd, Kulich S.M., Uechi G., Balasubramani M., Mountzouris J., Day B.W. Regulation of the autophagy protein LC3 by phosphorylation. J Cell Biol. 2010;190:533–539. doi: 10.1083/jcb.201002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang Y.W., Howard S.C., Herman P.K. The Ras/PKA signaling pathway directly targets the Srb9 protein, a component of the general RNA polymerase II transcription apparatus. Mol cell. 2004;15:107–116. doi: 10.1016/j.molcel.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 51.Budovskaya Y.V., Stephan J.S., Deminoff S.J., Herman P.K. An evolutionary proteomics approach identifies substrates of the cAMP-dependent protein kinase. Proc Natl Acad Sci U. S. A. 2005;102:13933–13938. doi: 10.1073/pnas.0501046102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torres-Quiroz F., Filteau M., Landry C.R. Feedback regulation between autophagy and PKA. Autophagy. 2015;11:1181–1183. doi: 10.1080/15548627.2015.1055440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galley Y., Hagens G., Glaser I., Davis W., Eichhorn M., Dobbelaere D. Jun NH2-terminal kinase is constitutively activated in T cells transformed by the intracellular parasite Theileria parva. Proc Natl Acad Sci USA. 1997;94:5119–5124. doi: 10.1073/pnas.94.10.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaussepied M., Lallemand D., Moreau M.F., Adamson R., Hall R., Langsley G. Upregulation of Jun and Fos family members and permanent JNK activity lead to constitutive AP-1 activation in Theileria-transformed leukocytes. Mol Biochem Parasitol. 1998;94:215–226. doi: 10.1016/s0166-6851(98)00070-x. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Q., Kuang H., Chen C., Yan J., Do-Umehara H.C., Liu X.Y. The kinase Jnk2 promotes stress-induced mitophagy by targeting the small mitochondrial form of the tumor suppressor ARF for degradation. Nat Immunol. 2015;16:458–466. doi: 10.1038/ni.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shiels B.R., McDougall C., Tait A., Brown C.G. Identification of infection-associated antigens in Theileria annulata transformed cells. Parasite Immunol. 1986;8:69–77. doi: 10.1111/j.1365-3024.1986.tb00834.x. [DOI] [PubMed] [Google Scholar]