Fig. 8.
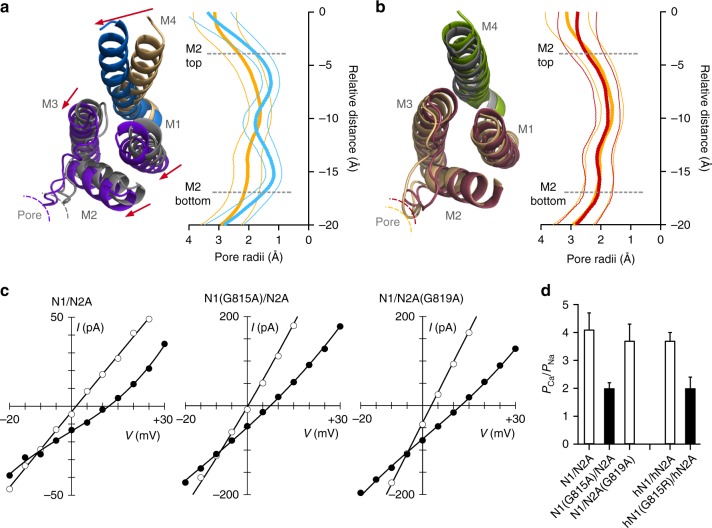
Constraining the conserved G in GluN1 reduces the dimensions of the selectivity filter and Ca2+ permeability. a Left, Cytoplasmic view of model open state structures of wild type and GluN1(G815A). The constrained M4 collapses the adjacent GluN2B ion channel core (M1–M3) including the pore size as defined by the M2 loop. Right, Average pore radius along the channel axis for the M2 pore loop for wild type (gold) and GluN1(G815A) (light blue). ’0’ references the serine (S) in SYTANLAAF in M3. Thick lines are mean values and thinner lines the error bars, which were calculated using block averages based on a time block of 20 ns. b Same as a except construct is GluN2B(G820A) (maroon), which resulted in a less displacement of the M2 loop. c Current-voltage (IV) relationships in an external solution containing high Na+ (140 mM) and 0 added Ca2+ (open circles) or 10 mM Ca2+ (solid circles). The 0 Ca2+ IV is the average of that recorded before and after the 10 mM Ca2+ recording. We used changes in reversal potentials (ΔErev) to calculate PCa/PNa37. d Mean ± SEM showing relative calcium permeability (PCa/PNa) calculated from ΔErevs for rat GluN1/GluN2A (n = 7), GluN1(G815A) (n = 8), or GluN2A(G819A) (n = 7) as well as human GluN1/GluN2A (n = 11) and the missense mutation GluN1(G815R) (n = 7). Values either are not (open bars) or are (solids bars) significantly different from each other (p < 0.05, ANOVA, Tukey)