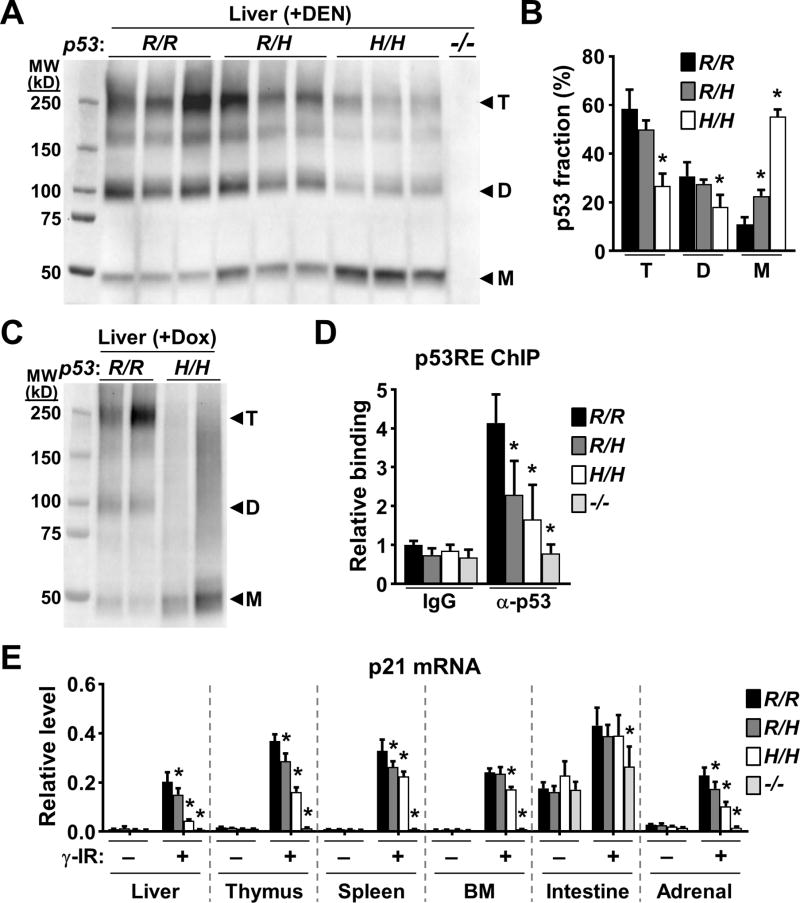
Figure 6.
p53 oligomerization is decreased in p53334H/H mouse tissues. A, liver tissue lysates were cross-linked with glutaraldehyde, resolved in SDS-PAGE gel, and immunoblotted. Note p53 antibody specificity demonstrated by lack of immunoreactivity in the p53−/− sample. Protein standards are in kD. Triplicate lanes of each genotype represent liver samples from 3 separate mice. B, fraction of p53 oligomers (T, tetramer; D, dimer; M, monomer) within each lane of immunoblot (A) quantified by densitometry and compared with the respective wild-type oligomer (n = 3). C, p53 immunoblot of cross-linked liver lysates obtained from mice 6 h after p53 induction by doxorubicin treatment. Duplicate lanes of each genotype represent liver samples from 2 separate mice. D, p53 binding to the p53 response element (p53RE) of p21 in γ-irradiated (γ-IR) mouse liver. ChIP was performed using nonspecific IgG or anti-p53 antibody. p53RE binding is shown relative to wild-type non-specific IgG samples (n = 3). E, induction of p21 mRNA in the indicated tissues by γ-IR quantified by RT-PCR (n = 3). Levels were normalized relative to a housekeeping gene TIF. Bone marrow (BM); small intestine. p53 R334 genotypes: wild-type (R/R); heterozygous mutant (R/H); homozygous mutant (H/H); and p53 null (−/−). Values are mean ± SD. Compared to wild-type samples within each group, statistical differences were tested by 1-way ANOVA. *P < 0.05.