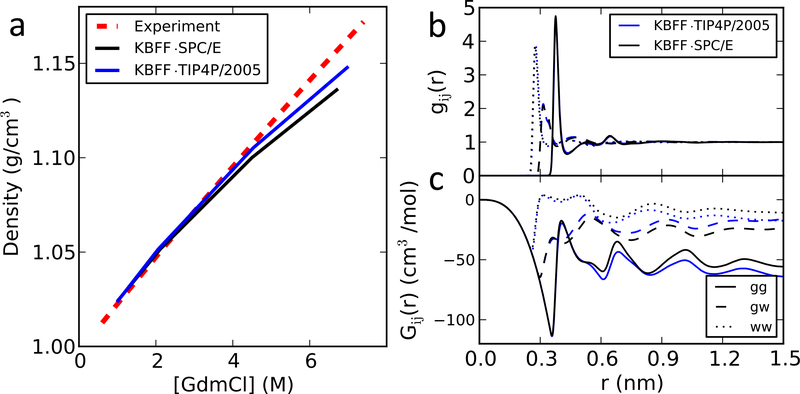
Figure 2:
Properties of GdmCl soluitons. (a) Density as a function of denaturant concentration; (b) radial distribution functions gij and (c) Kirkwood-Buff intergrals Gij of a 6M GdmCl solution. g and G between GdmCl and GdmCl are shown with solid lines; between GdmCl and water with dash lines; and between water and water with dotted lines. The values of KB integrals are the average between 0.95 and 1.3 nm for urea and between 1.10 and 1.45 nm for GdmCl, which were used in the KBFF work.12,13