Figure 8.
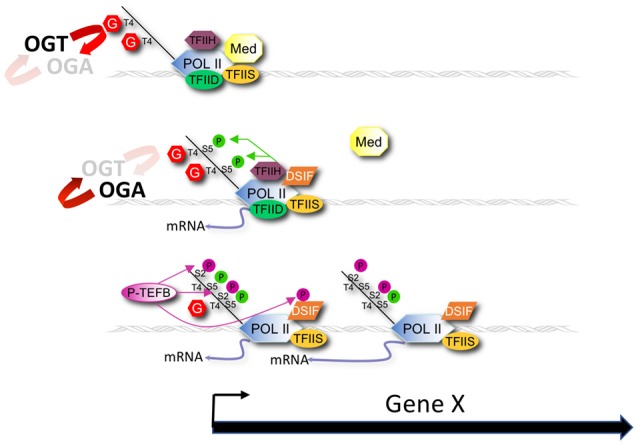
Modeling promoter region O-GlcNAcylation and Pol II dynamics. A model for the role of O-GlcNAc cycling in promoter proximal Pol II dynamics during nutrient flux based on our results is shown that also draws from earlier studies of Lewis and colleagues (37–40). OGT and OGA enzymatic activity dynamically cycles O-GlcNAc on Pol II, and other chromatin substrates, that localize at the promoter of many, if not all, genes. O-GlcNAcylation of the Pol II CTD competes with phosphorylation of Ser-2 and Ser-5 residues of the heptad repeats that are required for transcriptional initiation and elongation. Nutrition (e.g., fed condition) drives increased O-GlcNAcylation of Pol II that inhibits progression of the Pol II transcription cycle. Conversely, starvation decreases O-GlcNAcylation of Pol II, thus promoting transcription events. The consequence of the nutrient-sensitive O-GlcNAc cycle is a near constant, or buffered, level of promoter proximal Pol II. In the absence of O-GlcNAc activity (ogt-1 mutant) this buffering system is lost, resulting in Pol II dis-regulation. In the presence of excess O-GlcNAcylation (oga-1 mutant), the inhibitory effects of this modification on Pol II are only slightly greater than in wild type animals, thus minimally affecting Pol II dynamics.
