Figure 1.
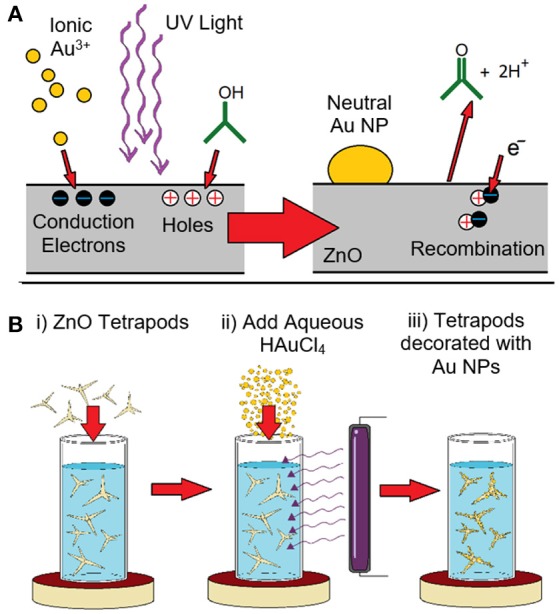
(A) Schematic representation of the light-mediated growth mode used to decorate ZnO tetrapods with Au nanoparticles. The incident UV light excites electron-hole pairs in the ZnO. The electrons excited to the conduction band reduce Au3+ ions which leads to Au deposition. The holes in the valence band are scavenged through recombination with electrons derived from a reaction which sees the oxidative reduction of isopropyl alcohol to acetone as well as the release of H+ ions into the solution. (B) Schematic showing the synthesis process in which (i) ZnO tetrapods are added to isopropyl alcohol and stirred, (ii) the reaction is initiated through the addition of aqueous HAuCl4 and the application of UV light, and (iii) Au nanoparticles (NPs) form on the ZnO tetrapods.
