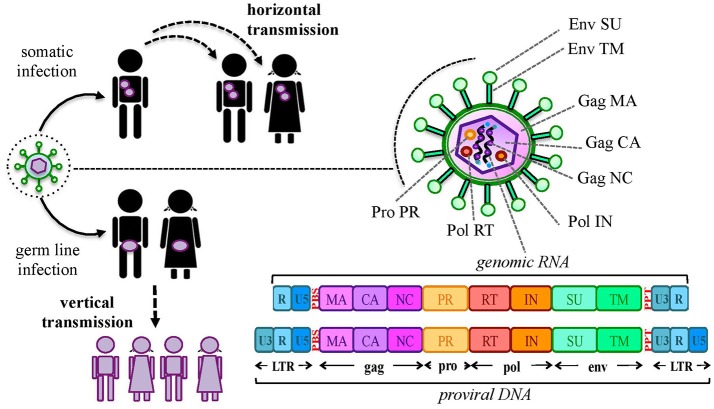
Figure 1.
Origin and general structure of HERV sequences. Exogenous retroviruses normally infect a specific type of somatic cells, being diffused from a host to new individuals by a horizontal transmission. In the case of HERVs, the ancestral retroviral infection affected the germ line cells: in this way, the proviral sequences have been endogenized and vertically transmitted to all the cells of descendant individuals. HERVs have been so inherited in a Mendelian fashion to the offspring, being fixed in the human population. The general structure of a full-length HERV provirus is represented: the two Long Terminal Repeats (LTRs) are formed during the reverse transcription of the viral RNA genome and flank the gag, pro, pol, and env genes. The primer binding site (PBS) and the polypurine trait (PPT) are located between 5′LTR and gag and between env and 3′LTR, respectively. The viral genes encode for the structural and non-structural proteins found in the viral particle: gag matrix (MA), capsid (CA) and nucleocapsid (NC); pro-pol protease (PR)—reverse transcriptase (RT) and integrase (IN); env surface (SU) and transmembrane (TM) subunits. While in exogenous retroviral infections the integrated provirus is transcribed by the cellular machinery to release new virions, the HERV persistence within the host genome and the action of cellular editing systems led to the accumulation of mutations that often made the proviruses coding-defective and thus unable to produce infectious particles.