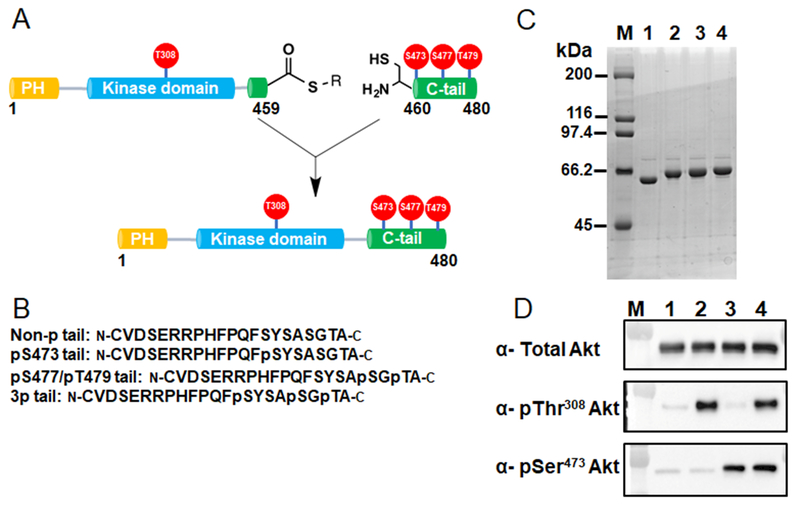
Figure 1. Semisynthesis of site-specifically phosphorylated Akt1 constructs.
A) Semisynthesis strategy for C-terminally phosphorylated Akt1 proteins with highlighted phosphorylations (red balls) that are studied in this work. C-terminally truncated recombinant Akt1 containing PDK1-catalyzed pThr308 and a C-terminal intein generated thioester is ligated to N-Cys synthetic C-tail peptides without or with phosphorylations at residues Ser473, Ser477 and Thr479. B) Representative C-terminal tail synthetic peptide sequences containing distinct phosphorylation states. C) Coomassie stained SDSPAGE of selected purified pThr308 containing Akt1 semisynthetic proteins: M, molecular weight standards, lane 1, unligated C-terminally truncated Akt1 MESNA thioester, lane 2, full-length FL-Akt1-pThr308, lane 3, FL-Akt1-pThr308/pSer473, lane 4, FL-Akt1-pThr308/3p-Ser473,Ser477,Thr479. D) Western blot analysis of selected purified semisynthetic Akt1 forms: M, MW markers, lane 1, non-pThr308, non-C-terminally phosphorylated full length Akt1, lane 2, FL-Akt1-pThr308, lane 3, FL-Akt1-pSer473, lane 4, FL-Akt1-pThr308/pSer473.