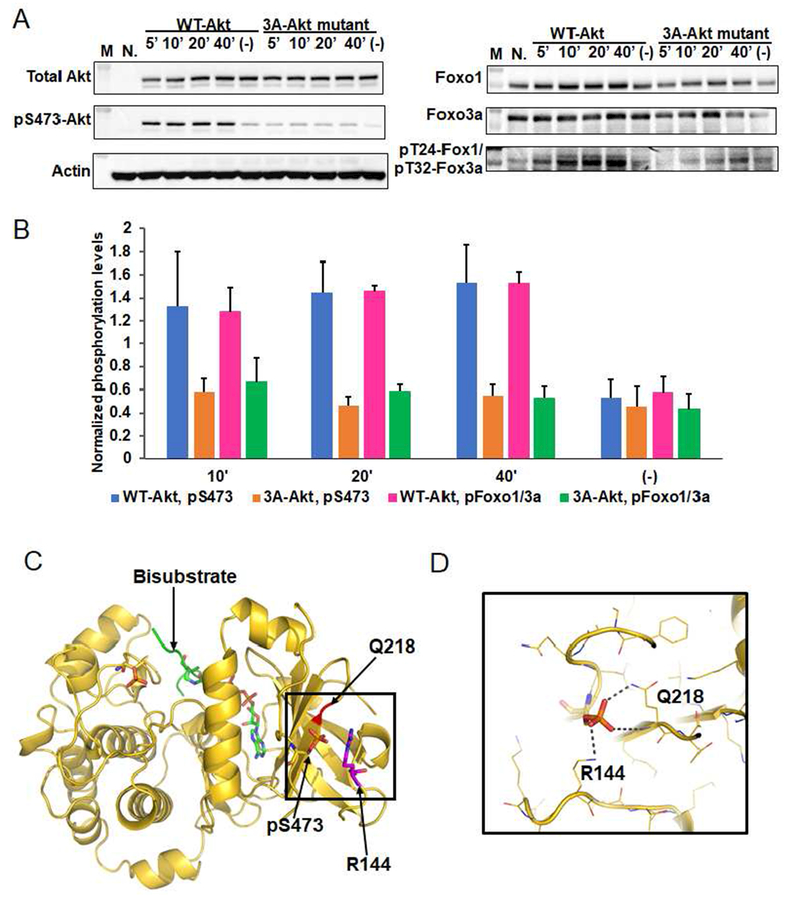
Figure 4. Phospho-Ser473 interacts with Arg144 in the PH-kinase linker basic patch.
A) Cellular analysis of the effect of basic patch on the Akt phosphorylation and activity. Akt1/2 knock-out HCT116 cells (Ericson et al., 2010) were transfected with pcDNA3 plasmids expressing wild type (WT) and K142A, H143A, R144A (3A) full-length Akt1s. After serum starvation, the cells were stimulated with growth factors for the time indicated. The cells were lysed and analyzed by western blot with Akt antibodies (left panel) and Foxo1 or Foxo3a antibodies (right panel). N.: non-transfected and stimulated with growth factors for 10 min., (−): transfected with DNA plasmids but not stimulated with growth factors; n=3 forassays. B) Quantification of phosphorylation level of Akt Ser473 (blue for WT Akt1 and orange for 3A-Akt1) and Foxo1 Thr24/Foxo3a Thr32 (pink for WT Akt1 and green for 3A-Akt1) using Image J (n=3, SEM shown, p<0.01) of blots represented by Figure 4A. C) X-ray structure of D122-Akt1-pThr308/3p-Ser473,Ser477,Thr479complexed with bisubstrate analog and residues Gln218, pSer473 and Arg144 are highlighted. D) Zoom-in of the region highlighted with a square in Figure 4C shows the interaction of pSer473 with Gln218 and Arg144, the pSer473 to Arg144 H-bond is 3.0 Å