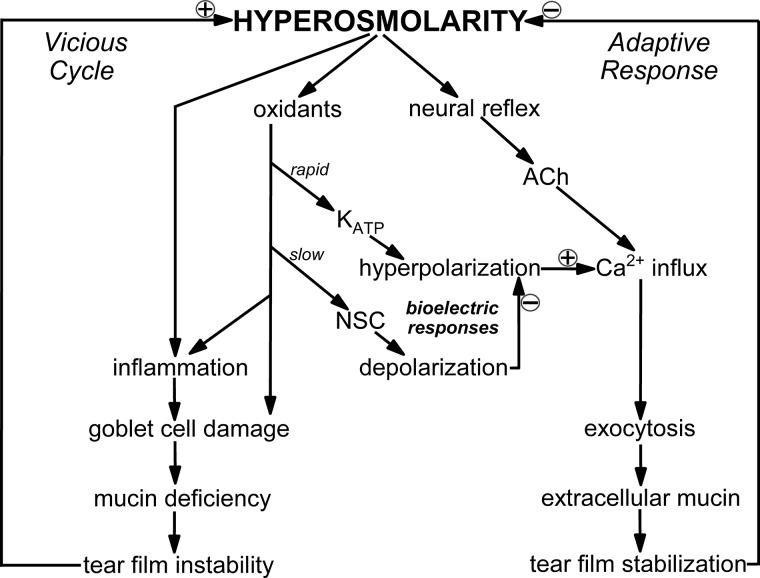
Fig. 9.
Model of bioelectric mechanisms by which conjunctival goblet cells respond to hyperosmolarity. A few minutes after a ≥10% rise in extracellular osmolarity, hyperpolarizing ATP-sensitive K+ (KATP) channels are activated via an oxidant-dependent mechanism. The resulting increase in voltage enhances the electro-gradient for Ca2+ influx and thereby boosts the exocytosis of tear-stabilizing mucin evoked by cholinergic input, which in vivo is triggered by a neural reflex and is chiefly driven by influxing Ca2+. The hyperpolarization-induced boost in the ability of goblet cells to release a bolus of tear-stabilizing mucin is a previously unappreciated mechanism by which the ocular surface effectively adapts to dryness/hyperosmolarity. However, during prolonged hyperosmolarity, development of a depolarizing nonspecific cation (NSC) conductance eventually fully counterbalances the KATP-driven hyperpolarization and thereby eliminates the boost in evoked exocytosis. Thus, the ability of goblet cells to release a bolus of mucin in response to cholinergic input becomes severely compromised during persistent hyperosmolarity. As illustrated in a highly simplified manner, unremitting hyperosmolarity activates stress pathways that if unchecked, activate a vicious cycle involving inflammation and ocular surface damage that can result in chronic dry eye.