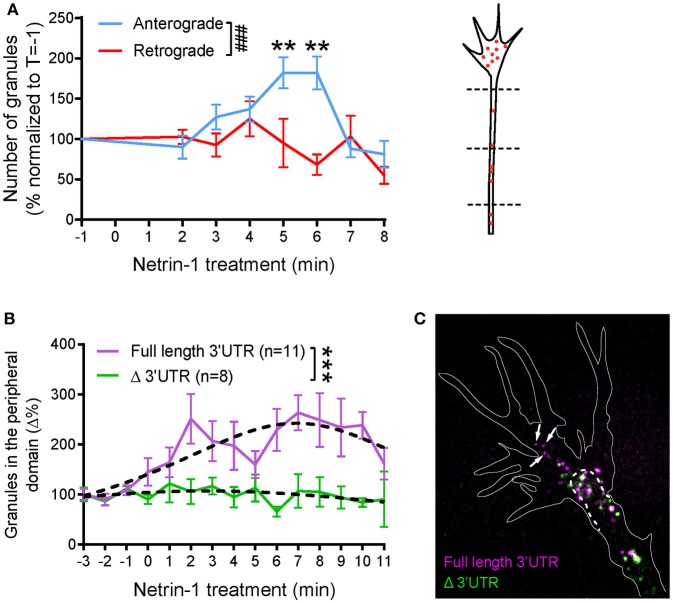
Figure 3.
Global netrin-1 stimulation changes the dynamics of β-actin mRNA granules. Axons were imaged at 1 frame per second from 1 min before bath application of netrin-1, and the imaging continued for up to 10 min after netrin application. (A) The numbers of β-actin mRNA granules passing three chosen locations (dashed lines) along the axon shaft were scored. The number of anterograde-moving granules increased upon netrin-1 treatment, reaching peak value at 5–6 min and returning to the base line by 8 min of netrin-1 treatment. The number of retrograde-moving granules showed moderate decrease with time upon netrin-1 treatment. (n = 9 axons) Anterograde vs. retrograde [F(7, 120) = 3.775, ###p = 0.001]; Vs. T = −1 min, **p < 0.01 (two-way ANOVA with Dunnett multiple-comparison test) (B–C) Global netrin-1 treatment enhanced β-actin mRNA localization to the growth cone periphery in a 3′UTR-dependent manner. (B) Quantification of mRNA granules localizing to the growth cone periphery. Netrin-1 treatment induced a marked increase in the number of full-length β-actin mRNA granules localizing to the growth cone periphery (n = 11 growth cones), a response that was abrogated for β-actin mRNA with truncated 3′UTR (n = 8 growth cones). Dotted lines represent least-squares fits to a Lorentzian function. [F(3, 178) = 22.01, ***p < 0.0001; extra sum-of-squares F test]. (C) Growth cones containing both full-length and Δ3′UTR β-actin mRNAs were fixed after 5-min bath netrin-1 treatment. The full-length mRNA granules localized further into the growth cone periphery (white arrows) while the Δ3′UTR granules remained in the central domain. Error bars represent SEM. See also Figure S2.