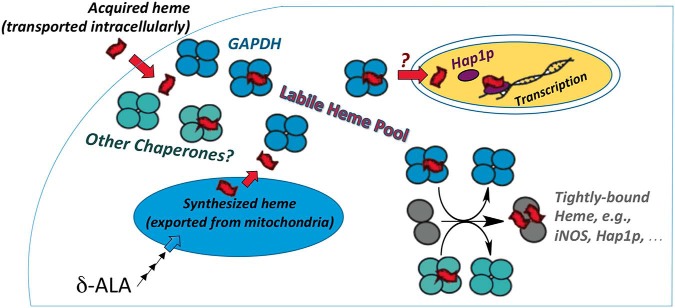
Figure 1.
Model of GAPDH as a heme chaperone that sequesters and transfers bioavailable heme to target hemeproteins. Heme can be synthesized endogenously from δ-ALA (aminolevulinic acid) or imported from exogenous sources through the plasma membrane. Sweeny et al. (2) demonstrate that GAPDH can bind both exogenous and endogenously generated heme and make heme bioavailable to target proteins. They also show that GAPDH-bound heme can be delivered to soluble proteins such as iNOS or to nuclear proteins such as the transcription factor Hap1p. There are likely heme chaperones other than GAPDH to interact with the many cellular target proteins.