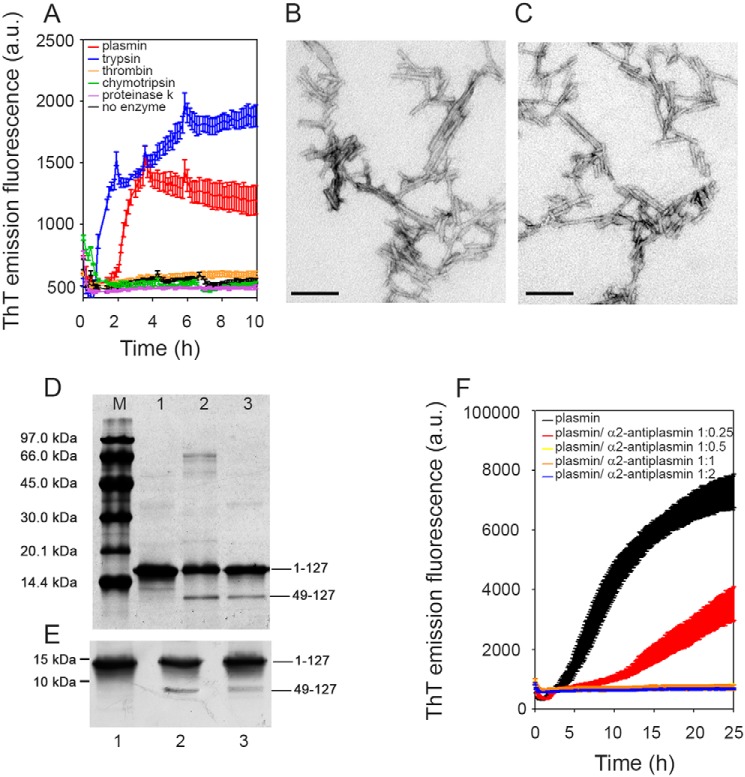
Figure 2.
Plasmin-mediated amyloid fibrillogenesis of S52P TTR. A, increase in ThT emission fluorescence for S52P TTR incubated in the presence of plasmin compared with trypsin. No amyloid-specific ThT signal was seen after incubation of S52P TTR with thrombin, chymotrypsin, or proteinase K. B and C, negatively stained transmission electron micrographs of S52P TTR amyloid fibrils formed in the presence of trypsin (B) or plasmin (C). Scale bar, 100 nm. D, 15% SDS-PAGE under reducing conditions. M, marker proteins (14.4, 20.1, 30.0, 45.0, 66.0, and 97.0 kDa); lane 1, S52P TTR at time 0; lane 2, S52P TTR fibrils formed in the presence of trypsin; and lane 3, S52P TTR fibrils formed in the presence of plasmin. E, immunoblot analysis of samples separated in 15% SDS-PAGE (see lanes 1, 2, and 3 in D). Position of marker proteins at 15 and 10 kDa are indicated. F, inhibition by α2-antiplasmin of fibril formation by S52P TTR mediated by 20 ng/μl plasmin. The data were normalized to the ThT signal plateau in the samples without α2-antiplasmin. Mean ± S.D. of three replicates is shown. a.u., arbitrary units.