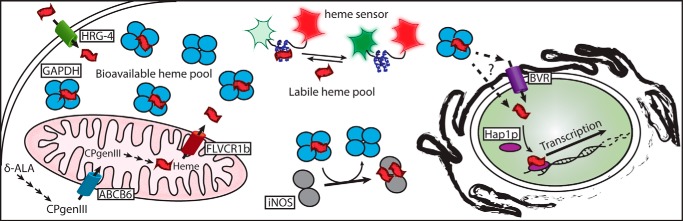
Figure 7.
Model of GAPDH as a cellular depot and allocator of bioavailable heme. Heme can be synthesized endogenously by the cell or imported through the plasma membrane. Endogenous heme biosynthesis occurs from glycine through the intermediates δ-ALA and coproporphyrinogen III (CPgenIII) catalyzed by enzymes found in the cytoplasm and mitochondria and finally exported into the cytoplasm by the transporter feline leukemia virus subgroup C receptor 1b (FLVCR1b). Exogenous heme can be imported into the cell through transporters such as histidine-rich glycoprotein 4 (HRG-4). GAPDH can bind both endogenous and exogenous heme sources thereby sequestering heme into a bioavailable pool. Heme binding to the heme sensor HS1 causes a decrease in EGFP (green) fluorescence but has no effect on mKATE (red) fluorescence. The labile heme pool detected by the heme sensor is distinct from the GAPDH-bound bioavailable heme pool. The GAPDH-bound heme can be delivered to soluble proteins such as iNOS or to nuclear proteins such as the transcription factor Hap1p. Heme transport into the nucleus may occur through a hand-off to the transporter of BVR or directly carried into the nucleus by GAPDH.