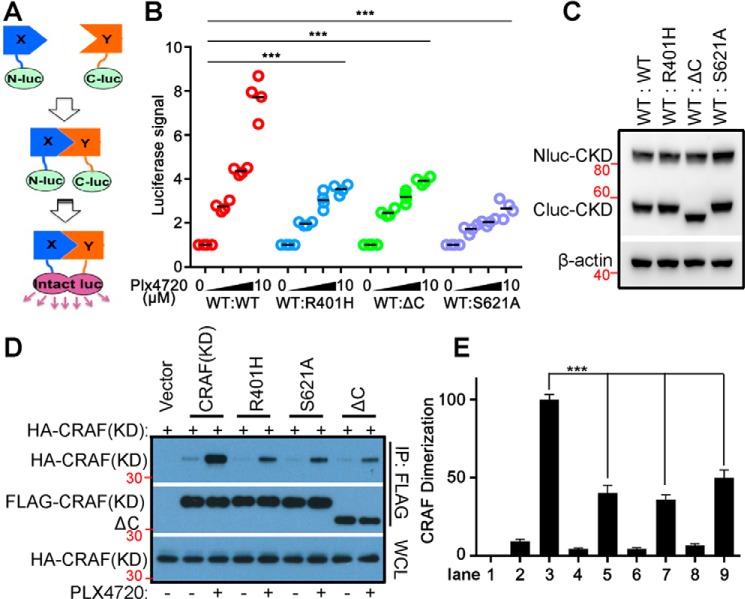
Figure 2.
Disruption of 14-3-3 binding to the C terminus of CRAF inhibits RAF inhibitor–induced dimerization. A–C, the dimer affinity of CRAF or its mutants was measured by complimentary split luciferase assay as detailed under “Experimental procedures.” A, a diagram illustrating the complimentary split luciferase assay. B, luciferase signals were measured to study the interaction between WT and mutant CRAFs (aa 322–648; R401H, S621A, and ΔC). Briefly, 293T cells co-expressing Nluc- or Cluc-fused CRAF and its mutants were treated with PLX4720 before the luciferase signals were measured using an illuminometer. Each dot represents the means from the technical repeat of triple wells in the same study (n = 4; ***, p < 0.001). C, the protein level of Nluc- and Cluc-CRAF fusion proteins in 293T cells from B was measured by immunoblot. D and E, the dimerization of CRAF or its mutants was evaluated by co-immunoprecipitation assay. HA-tagged CKD (aa 322–648) was co-expressed with FLAG-tagged CKD or its mutants in 293T cells. Then cells were treated with or without 10 μm PLX4720 for 2 h, and immunoprecipitation was carried out with anti-FLAG beads and lysis/washing buffer containing 0.1% NP-40 with or without 10 μm PLX4720. The HA- and FLAG-tagged CKD or mutants in immunoprecipitants were detected by immunoblots (D) and quantified by ImageJ, and their ratio was calculated (E) (n = 3; ***, p < 0.001). All images are representative of at least three independent experiments.