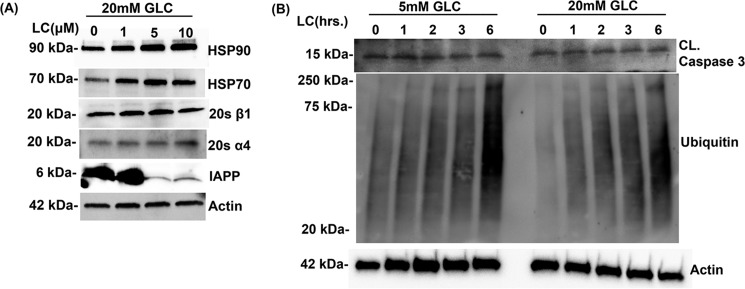
Figure 4.
Effect of proteasome inhibition on expression levels of heat-shock proteins, proteasome complex subunits, and the viability of human islet cells. A, Western blot analysis of whole-cell extracts of human islet cells cultured in 20 mm glucose (Glc)-containing media for 6 days followed by dose-dependent inhibition of proteasome activity (0–10 μm) with LC for 12 h. Blots were probed with anti-HSP90, anti-HSP-70, 20 α1, 20S β1, and anti-actin antibodies. B, Western blot analysis of whole-cell extracts of human islet cells following temporal (0–6 h) inhibition of proteasome activity with LC (10 μm). Blots were probed with anti-ubiquitin (protein stress marker) and anti-caspase 3 (apoptotic marker) and anti-actin (loading control) antibodies. The actin blot was reused in both this panel and Fig. 2A because proteins analyzed in these Western blottings were resolved on the same gel.