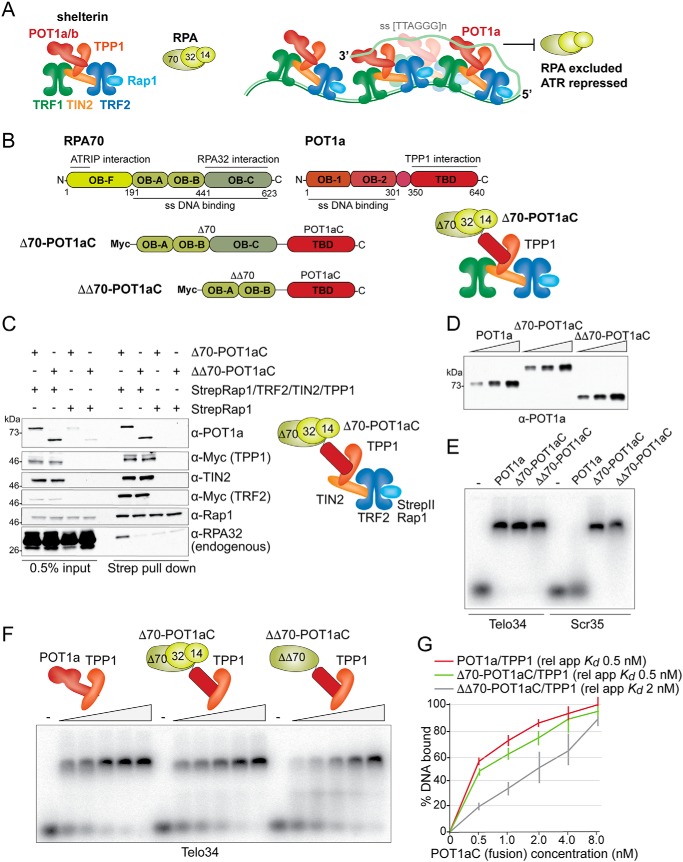
Figure 1.
Characterization of the RPA-POT1aC fusion proteins. A, schematic (left) of the mouse shelterin complex and RPA and model (right) for how the binding of POT1a to shelterin and ss TTAGGG repeats excludes RPA from telomeres and thereby prevents ATR activation. B, schematic of mouse RPA70, mouse POT1a and the two fusion proteins Δ70-POT1aC and ΔΔ70-POT1aC, indicating the amino acids and functions of the relevant domains. Right, schematic of mouse TPP1-tethered Δ70-POT1aC and RPA32 and RPA14. C, immunoblots showing co-IPs from transiently transfected 293T cells of Δ70-POT1aC or ΔΔ70-POT1aC with either Strep-tagged Rap1 or Strep-tagged Rap1 together with TRF2, TIN2, and TPP1. Antibodies to the expressed proteins and the endogenous RPA32 are indicated. D, example of immunoblotting to determine the relative protein concentration of Strep-tagged TPP1/POT1a or TPP1/RPA-fusion proteins isolated from transfected 293T cells. E, EMSA showing binding of TPP1/POT1a, TPP1/Δ70-POT1aC, and TPP1/ΔΔ70-POT1aC to telomeric and nontelomeric ssDNA. Telo34, 34 nts of single-stranded telomeric DNA; Scr35, 35 nts of single-stranded nontelomeric DNA (see “Experimental Procedures” for probe sequences). F, representative EMSA to determine the apparent relative Kds of TPP1/POT1a, TPP1/Δ70-POT1aC, and TPP1/ΔΔ70-POT1aC for Telo34. G, quantification of the affinity of TPP1/POT1a, TPP1/Δ70-POT1aC, and TPP1/ΔΔ70-POT1aC as shown in E from three independent experiments. Error bars represent S.D.s.