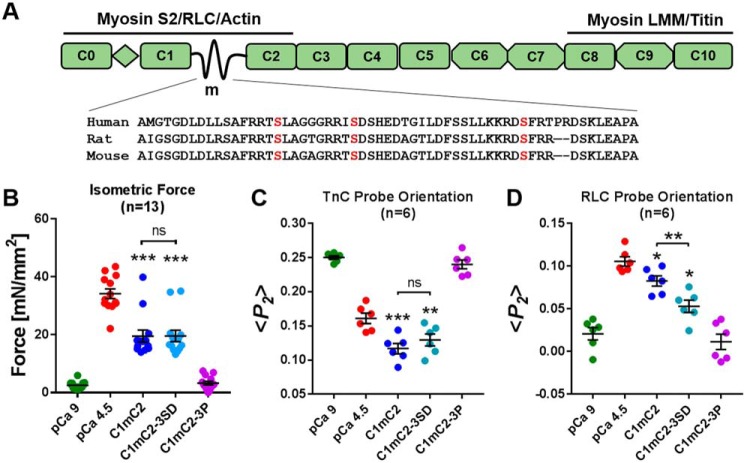
Figure 1.
Effect of phosphomimetic substitutions in cMyBP-C on isometric force and thin and thick filament structure in cardiac muscle. A, schematic of cMyBP-C's domain organization and known protein interactions, with a sequence alignment for the cardiac-specific m-motif containing the phosphorylatable serine residues. LMM, light meromyosin; RLC, regulatory light chain; myosin S2, myosin sub-fragment 2. B, isometric force. C, TnC probe orientation. D, RLC probe orientation of ventricular trabeculae in relaxing conditions (pCa 9, green), at full calcium activation (pCa 4.5, red), and during incubation in relaxing solution (pCa 9) containing 40 μmol/liter C1mC2 (blue), phosphomimetic C1mC2–3SD (turquoise), or PKA tris-phosphorylated C1mC2–3P (purple). Mean ± S.E. with the number of trabeculae (n) indicated in each panel. Statistical significance of differences between groups was assessed with a one-way ANOVA followed by Tukey's post hoc test: *, p < 0.05; **, p < 0.01; ***, p < 0.001, and ns, not significant.