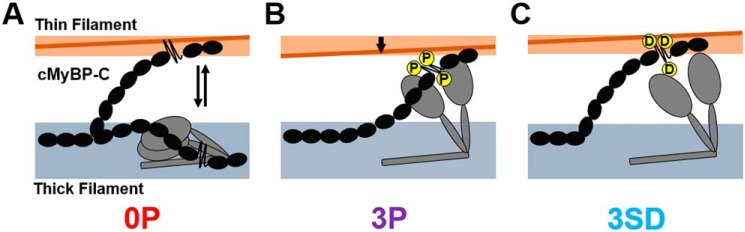
Figure 3.
Model for the effect of phosphorylation or serine-aspartate substitutions on cMyBP-C function. A, in the unphosphorylated state, cMyBP-C binds both thin and thick filaments stabilizing their ON and OFF states, respectively. cMyBP-C inhibits myosin head domains (gray) by stabilizing their folded OFF state and activates the thin filament by moving tropomyosin (brown) away from its blocked position toward the open position. B, PKA tris-phosphorylation (P) abolishes both the activating and inhibitory effects of cMyBP-C on the thin and thick filament, respectively. C, in contrast, serine-to-aspartate substitutions (D) abolish the inhibitory interaction between cMyBP-C and myosin but do not abolish the activating interaction with the thin filament.