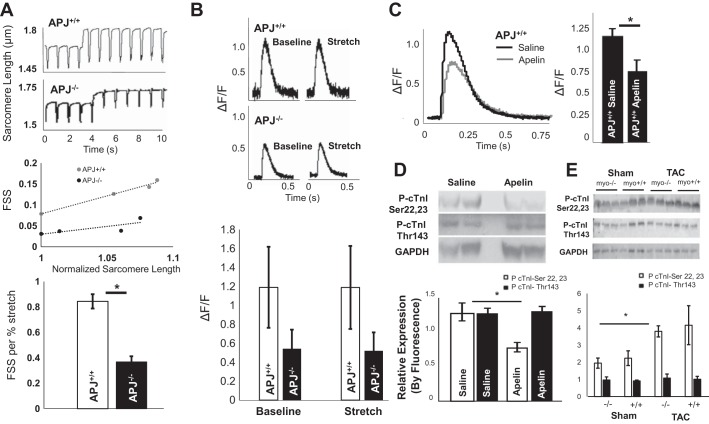
Fig. 3.
Apelin lowers Ca2+ transients in isolated cardiomyocytes and simultaneously increases myofilament sensitivity, whereas length-dependent activation by APJ is independent of Ca2+ transients. A: representative sarcomere length tracings and slopes of fractional sarcomere shortening (FSS) per increase in diastolic sarcomere length (stretch) [APJ−/− FSS/%: 0.37 ± 0.04/% sarcomere length (SL) and APJ+/+: 0.84 ± 0.05/% SL, P = 0.04 (mean slopes ± SE, n = 3 mice/group]. B: change in Ca2+-induced Ca2+ release in APJ−/− compared with APJ+/+ cardiomyocytes [APJ−/− ΔF/F: 1.2 ± 0.43 and APJ+/+ ΔF/F: 0.54 ± 0.2, P = 0.2 (means ± SE, n = 3 mice/group)]. No difference in ΔF/F was found within genotype between baseline and stretch. C: apelin significantly decreased peak Ca2+ transients in APJ+/+ cardiomyocytes (49% reduction in ΔF/F in apelin-treated animals, apelin: n = 39 cells from four animals and saline: n = 23 cardiomyocytes from four animals in the control group, P = 3 × 10−4). D: immunoblot analysis of cardiac troponin I (cTnI) phosphorylation in apelin- and saline-treated mice using anti-phospho (Ser22 and Ser23) antibody against the PKA-mediated site demonstrated decreased phosphorylation in the presence of apelin (P < 0.01). No difference was found in PKC-phosphorylated residue Thr143 (n = 6 per group). E: immunoblot showing fluorescence detection of antibodies to phospho-cTnI (Ser22 and Ser23), phospho-cTnI (Thr143), and GAPDH in cardiac tissue of APJmyo+/+ and APJmyo−/− mice before and after transaortic constriction (TAC). Fluorescence quantification of phospho-cTnI (Ser22 and Ser23, open bars) and phospho-cTnI (Thr143, solid bars) normalized to GAPDH fluorescence is shown (bars show means ± SD of arbitrary units). *P < 0.01.