Abstract
p21-activated kinases (PAKs) are highly conserved serine/threonine protein kinases, which are divided into two groups: group-I (PAKs1–3) and group-II (PAKs4–6). In various tissues, Group-II PAKs play important roles in cytoskeletal dynamics and cell growth as well as neoplastic development/progression. However, little is known about Group-II PAK’s role in a number of physiological events, including their ability to be activated by gastrointestinal (GI) hormones/neurotransmitters/growth factors (GFs). We used rat pancreatic acini to explore the ability of GI hormones/neurotransmitters/GFs to activate Group-II-PAKs and the signaling cascades involved. Only PAK4 was detected in pancreatic acini. PAK4 was activated by endothelin, secretagogues-stimulating phospholipase C (bombesin, CCK-8, and carbachol), by pancreatic GFs (insulin, insulin-like growth factor 1, hepatocyte growth factor, epidermal growth factor, basic fibroblast growth factor, and platelet-derived growth factor), and by postreceptor stimulants (12-O-tetradecanoylphobol-13-acetate and A23187). CCK-8 activation of PAK4 required both high- and low-affinity CCK1-receptor state activation. It was reduced by PKC-, Src-, p44/42-, or p38-inhibition but not with phosphatidylinositol 3-kinase-inhibitors and only minimally by thapsigargin. A protein kinase D (PKD)-inhibitor completely inhibited CCK-8-stimulated PKD-activation; however, stimulated PAK4 phosphorylation was only inhibited by 60%, demonstrating that it is both PKD-dependent and PKD-independent. PF-3758309 and LCH-7749944, inhibitors of PAK4, decreased CCK-8-stimulated PAK4 activation but not PAK2 activation. Each inhibited ERK1/2 activation and amylase release induced by CCK-8 or bombesin. These results show that PAK4 has an important role in modulating signal cascades activated by a number of GI hormones/neurotransmitters/GFs that have been shown to mediate both physiological/pathological responses in acinar cells. Therefore, in addition to the extensive studies on PAK4 in pancreatic cancer, PAK4 should also be considered an important signaling molecule for pancreatic acinar physiological responses and, in the future, should be investigated for a possible role in pancreatic acinar pathophysiological responses, such as in pancreatitis.
NEW & NOTEWORTHY This study demonstrates that the only Group-II p21-activated kinase (PAK) in rat pancreatic acinar cells is PAK4, and thus differs from islets/pancreatic cancer. Both gastrointestinal hormones/neurotransmitters stimulating PLC and pancreatic growth factors activate PAK4. With cholecystokinin (CCK), activation is PKC-dependent/-independent, requires both CCK1-R affinity states, Src, p42/44, and p38 activation. PAK4 activation is required for CCK-mediated p42/44 activation/amylase release. These results show PAK4 plays an important role in mediating CCK physiological signal cascades and suggest it may be a target in pancreatic acinar diseases besides cancer.
Keywords: bombesin, CCK, celluar calcium, pancreatic acini, protein kinase C, signaling
INTRODUCTION
The p21-activated kinases (PAKs) are a highly conserved family of serine/threonine protein kinases that are one of the main effectors for the small Rho GTPases, cell division control protein 42 homolog (Cdc42), and Ras-related C3 botulinum toxin substrate (Rac) (42, 57, 89). The PAK family consists of six members that are structurally divided in two subgroups: (Group-I, PAK1–3 and Group-II, PAK4–6) based on sequence homology (41, 42, 83, 89). Group-I PAKs are the most extensively studied and play an important role in signaling for many cellular processes, such as regulation of cell survival, apoptosis, cell motility, tumorigenesis, protein synthesis, glucose homeostasis, secretion, and cellular proliferation (11, 41, 49, 81, 89). Group-II PAKs play important roles in a number of fundamental cellular processes, and PAK4 is the most extensively studied member of the Group-II PAKs (17, 26, 44, 51). Group-II PAKs have been shown to be particularly important in the regulation of cell morphology, cytoskeletal organization, cell proliferation, cell cycle control, migration, and survival (1, 17, 26, 44). They have also been extensively studied in neoplastic processes, with overexpression, particularly PAK4, found in a number of cancers, including cancers of the pancreas, prostate, gall bladder, stomach, and gastrointestinal (GI) (42, 64, 83, 84); PAK4 overexpression can trigger oncogenic transformation in normal cells, and PAK4 signaling is important in tumor progression (37, 42, 64). However, little is known of the role of Group-II kinases in a number of physiological processes. One such physiological process is their role in signaling by GI hormones/neurotransmitters and GI growth factors (GFs) in normal GI tissues. Pancreatic acinar cells possess a number of specific receptors for GI hormones/neurotransmitters/GFs and are highly responsive to these stimuli and therefore are an excellent model to investigate the role of PAK4 in their signaling (10, 34, 49). Recent studies (51, 74) using this approach have demonstrated that the Group-I PAK, PAK2, can be activated in pancreatic acini by a number of these stimuli and can modulate signaling cascades involved in both physiological and pathological responses.
To address the above questions in the case of the Group-II PAK4, in the present study we first sought to determine whether the three members of the Group-II PAKs were present in rat pancreatic acinar cells and then, if present, whether they are activated by GI hormones/neurotransmitters or by various GI GFs, which are known to alter pancreatic acinar cell function. We also studied in detail the signaling cascades for cholecystokinin (CCK), a physiological regulator of pancreatic acinar cells (20), to activate PAK4 and the role of CCK’s activation of PAK4 in stimulating pancreatic secretion and known growth cascades.
MATERIALS AND METHODS
Materials
Male Sprague-Dawley rats (150–250 g) were obtained from the Small Animals Section of the Veterinary Resources Branch of the National Institutes of Health (Bethesda, MD). AR42J cells (rat pancreatic acinar cancer cells) were from the American Type Culture Collection (Rockville, MD). PAK1 recombinant protein was from Abnova (Walnut, CA). Recombinant PAK2 protein was from Active Motif (Carlsbad, CA). PAK3 recombinant human protein was from Life Technologies Corporation (Grand Island, NY). PAK4 recombinant human protein was from MyBiosource (San Diego, CA). Phospho-PAK4 (S474)/PAK5 (S602)/PAK6 (S560) was from GeneTex (Irvine, CA). Phospho-PAK1 (Thr423)/PAK2 (Thr402), PAK2, Phospho-p44/42 MAPK (Thr202/Tyr204), p44/42 MAPK, protein kinase D (PKD) (S916), and PKD antibodies were from Cell Signaling Technology (Beverly, MA). Stabilized goat anti-rabbit IgG peroxidase conjugated was from Pierce Biotechnology (Rockford, IL). PAK4 (P-21) and antigoat horseradish peroxidase-conjugate antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). PAK5 antibody was from Biorbyt (San Francisco, CA). PAK6 antibody was from BioVision (Milpitas, CA). Tris·HCl pH 8.0 and 7.5 were from Mediatech (Herndon, VA). Two-mercaptoethanol, protein assay solution, sodium lauryl sulfate, and Tris-glycine-sodium lauryl sulfate (×10) were from Bio-Rad (Hercules, CA). MgCl2, CaCl2, Tris·HCl 1 M pH 7.5, and Tris-glycine buffer (×10) were from Quality Biological (Gaithersburg, MD). PAK6 recombinant protein, minimal essential media vitamin solution, amino acids ×100, Dulbecco's phosphate-buffered saline, glutamine (200 mM), 4–20% Tris-glycine gels, L-glutamine, fetal bovine serum, ethidium bromide solution, and SuperScript III First-Strand Synthesis SuperMix for qRT-PCR were from Invitrogen (Carlsbad, CA). COOH-terminal octapeptide of CCK-8, hepatocyte growth factor (HGF), bombesin, insulin-like growth factor 1 (IGF-1), basic fibroblast growth factor (bFGF), endothelin (ET), and secretin were from Bachem Bio-Science (King of Prussia, PA). CCK-JMV-180 (CCK-JMV) was obtained from Research Plus (Barnegat, NJ). Epidermal growth factor (EGF), thapsigargin (TG), platelet-derived growth factor (PDGF), vascular endothelial growth factor, PAK5 active protein, and deoxycholic acid were from Calbiochem Corp. (La Jolla, CA). Carbachol, insulin, dimethyl sulfoxide, 12-O-tetradecanoylphobol-13-acetate (TPA), l-glutamic acid, glucose, fumaric acid, pyruvic acid, trypsin inhibitor, HEPES, Tween 20, Triton X-100, GFX109203X (GFX), phenylmethanesulfonylfluoride, ethylenediaminetetraacetic acid, ethylene glycol tetraacetic acid, sucrose, sodium orthovanadate, sodium azide, CRT0066101, and kbNB142-70 were from Sigma-Aldrich (St. Louis, MO). Albumin standard and SuperSignal West (Pico, Dura) chemiluminescent substrate were from Pierce Biotechnology. Protease inhibitor tablets were from Roche Holding AG (Basel, Switzerland). Purified collagenase (type CLSPA) was from Worthington Biochemical (Freehold, NJ). Nitrocellulose membranes were from Schleicher and Schuell Bioscience (Keene, NH). L-364,718[3S(−)-N-(2,3-dihydro-1-methyl-2-oxo-5-phenyl-1H-1,4-benzodiazepine-3-yl-1H-indole-2-carboxamide)] was from Merck, Sharp, and Dohme (West Point, PA). YM022 [(R)-1-[2,3-dihydro-1-(2′-methyl-phenacyl)-2-oxo-5-phenyl-1H-1,4-benzodiazepin-3-yl]-3-(3-methylphenyl)urea] was from Tocris Bioscience (Ellisville, MO). Albumin bovine fraction V was from MP Biomedicals (Solon, OH). NaCl, KCl, and NaH2PO4 were from Mallinckrodt (Paris, KY). RNeasy Mini Kit, DNase Digestion, and HotStarTaq Master Mix Kit were from Qiagen (Valencia, CA). NuSieve 3:1 Agarose was from Lonza (Walkersville, MD). PAK4, PAK5, and PAK6 primers were from Integrated DNA Technology (Coralville, IA). Rat brain RNA was from ZYAGEN (San Diego, CA). Nonfat milk Ominlok was purchased from AmericanBio (Natick, MA). PF-3758309, PD98059, and SB202190 were from Apexbio Technology LLC (Houston, TX). Phadebas Amylase Test was from Magle Life Sciences (Cambridge, MA). Adenovirus Rapid Quantification Kit and ViraBind Adenovirus Purification Kit were form Cell Biolabs (San Diego, CA). PAK4 Adenovirus, Null Adenovirus, and ViralPlus Transduction Enhancer were from Applied Biological Materials (Richmond, Canada). N2-(3-methoxyphenyl)-N4-[(oxolan-2-yl)methyl]quinazoline-2,4-diamine(LCH-7749944) was from MolPort (Riga, Latvia).
Methods
Pancreatic acini preparation.
All animal experiments were approved by the Animal Ethics Committee of the National Institutes of Health and carried out in accordance with the International Guiding Principles for Animal Research. Pancreatic acini preparation was obtained by collagenase digestion, and acinar cell incubation conditions were as previously described (49, 70, 77).
Pancreatic acini stimulation.
After collagenase digestion, dispersed acini were preincubated in standard incubation solution for 2 h at 37°C as described previously (49, 70). After preincubation, 1-ml aliquots of dispersed acini were incubated at 37°C with or without stimulants, and then cells were lysed, sonicated, and lysates were centrifuged at 10,000 g for 15 min at 4°C as described previously (49, 70). Protein concentration was measured using the Bio-Rad protein assay reagent.
RNA isolation and nonquantitative RT-PCR.
Total RNA was isolated from frozen rat brain (ZYAGEN), pancreatic acinar cells, and AR42J cells. Total RNA was prepared using a RNeasy Mini Kit (Qiagen). RNA samples were treated with DNase Digestion (Qiagen) during preparation to remove contaminating DNA. Total RNA (1 μg) was reverse transcribed using a SuperScript III First-Strand Synthesis SuperMix for qRT-PCR (Invitrogen) according to the manufacturer’s instructions for complementary DNA synthesis. PCR (primers for PAK4, PAK5, and PAK6) was selected through analysis of the rat PAK4, PAK5, and PAK6 mRNA sequence (GenBank accession no. NM_001106238, NM_001107781, and NM_001106498, respectively). The sense and antisense sequences of the primer were as follows: PAK4, sense, 5′-GCAGCTAGGCCGCGAG-3′ (nucleotides 75–90) and antisense, 5′-CAGGCACCTGGTCTGAAGTG-3′ (nucleotides 189–170), giving a PCR product size of 115 bp; PAK5, sense, 5′-AGCCGTAGTAGTTCCCCAGC-3′ (nucleotides 157–176) and antisense, 5′-CTGACGATTGTCTTCATGGGAGC-3′ (nucleotides 788–766), giving a PCR product size of 632 bp; and PAK6, sense, 5′-CTTCTAACTCTCCCCGCCCTA-3′ (nucleotides 106–126) and antisense, 5′-TACTACCGTCTTCATGGGCTGC−3′ (nucleotides 849–828), giving a PCR product size of 744 bp. The presence of the PAK’s (PAK4, PAK5, and PAK6) mRNA was determined in complementary DNA samples from rat brain, pancreatic acinar, and AR42J cells. Amplification for all PCR reactions included an initial cycle of 95°C for 15 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 60°C for 30 s and extension at 72°C for 1 min. After the final cycle, all PCR reactions concluded with 10 min extension at 72°C. PCR products were size fractionated on 3% agarose gels, stained with ethidium bromide, and visualized under UV light.
Inhibition experiments.
Preincubation with two different classes of PAK4 inhibitors, PF-3758309 and LCH-7749944 (48, 60, 87), was performed (49, 51) to identify downstream effects of CCK-8-mediated activation of PAK4. Isolated acini were preincubated for 1 h or 3 h with PF-3758309 or LCH-7749944 and then treated for 3 min with 1 nM CCK-8 or 5 min with 1µM TPA. Untreated cells were used as controls. After incubation, cells were processed as below in Western blot analysis.
Western blot analysis.
Western blot analysis was performed as described previously (24, 62). Briefly, cell lysates were separated on 4–20% Tris-glycine gels and transferred to nitrocellulose membranes. After blocking the membranes, they were incubated with primary antibody at antibody dilutions suggested by the supplier and incubated with horseradish peroxidase-conjugated secondary antibody (anti-mouse, anti-rabbit, and anti-goat) according to the species of the first antibody as described previously (49, 51). Protein bands were measured using GeneTools software from Syngene (Frederick, MD), which were assessed in the linear detection range.
Amylase release.
Amylase release was performed as described previously (49, 62, 66). Amylase activity was determined after 30 min incubation using the Phadebas reagent and was expressed as percentage of the total cellular amylase released into the extracellular medium during the incubation (49, 62, 66).
Adenovirus infection.
To examine the effects of overexpression of PAK4, and to determine the opposite effect of the pharmacological inhibition of PAK4, we used a PAK4 adenovirus (PAK4 Advirus) (18) and null Advirus (as an infection control). Both viral constructs were amplified and quantified as previously described (6). Isolated acini were infected for 12 h with either null Advirus (empty adenovirus, as infection control) or PAK4 Advirus at 1 × 109 VP/ml concentration (6). After 12 h, cells were lysed as described in Western blot analysis.
Statistical analysis.
Data from at least three different experiments are presented as means ± SE and were analyzed with the nonparametric Kruskal-Wallis analysis (because the data was not normally distributed) using the GraphPad Prism 6.0 software. P values < 0.05 were considered significant.
RESULTS
Presence of PAK4 mRNA and protein in pancreatic acini and specificity of antibodies used in the study.
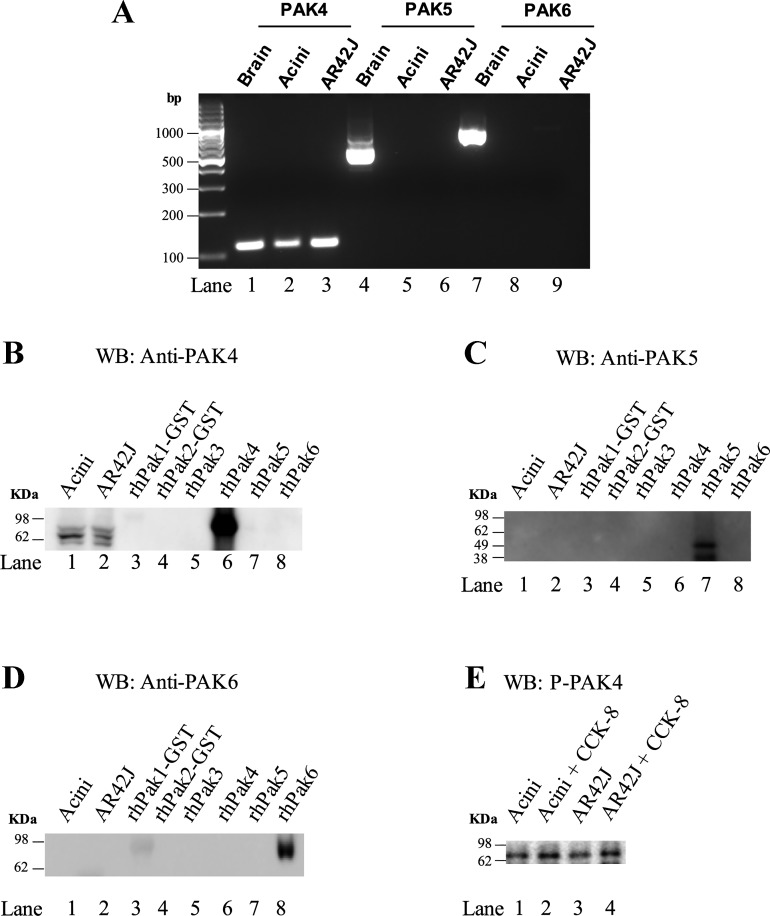
A previous study established that PAK2 is the only member of the Group-I PAKs expressed in rat pancreatic acini (49). To determine if the p21-activated kinase Group-II PAKs (PAK4, 5, and 6) are present in pancreatic acini, both studies of mRNA and protein of Group-II PAKs were determined (Fig. 1). In rat pancreatic acinar cells and in the rat pancreatic acinar cell line, AR42J, only a single band of 115 bp for PAK4 was found (Fig. 1A, Lanes 1–3). To determine if PAK4 protein was also expressed, a Western blot was performed with different human recombinants of the 6 PAK family members’ cells running in parallel with lysates obtained from rat pancreas acini and AR42J cells using antibodies specific for PAK4, PAK5, and PAK6 (Fig. 1, B–D). We confirmed that each of the three antibodies was specific for their respective PAK-II subtype. Only PAK4 protein was found in pancreatic acinar cells and AR42J cells (Fig. 1B, Lanes 1–2). Moreover, using a phospho-S474 PAK4 antibody in pancreatic acinar cells and in AR42J cells stimulated with CCK-8 at 1 nM, an increase in S474 phosphorylation of PAK4 was observed (145 ± 13% and 139 ± 17% of control, respectively. P < 0.05 vs. control) (Fig. 1E). These results demonstrate that the only Group-II PAKs present in pancreatic acini is PAK4 and that it can be activated by the physiological stimulant CCK-8.
Fig. 1.
Specificity of p21-activated kinase (PAK)4, 5, and 6 antibodies and demonstration that pancreatic acini and AR42J cells possess only PAK4 mRNA/protein. Representative PCR results for PAK4, PAK5, and PAK6 mRNA in rat brain, pancreatic acinar cells, and AR42J cells (A). Lysates from pancreatic acinar cells and equal amounts (5 µg) of human recombinant PAK family members (PAK1–6) or AR42J cells were detected by Western blot analysis (WB) with specific anti-PAK4 (Santa Cruz Biotechnology) (B), anti-PAK5 (Biorbyt) (C), and anti-PAK6 (BioVision) (D) antibodies, or with a specific antiphospho PAK4 antibody (GeneTex) (E). Results from pancreatic acini or AR47J cells with or without 1 nM CCK-8 stimulation (E). These results are representative of 4 other experiments. CCK-8, cholecystokinin-8.
Stimulation of PAK4 phosphorylation by various pancreatic secretagogues and pancreatic GFs in rat pancreatic acinar cells.
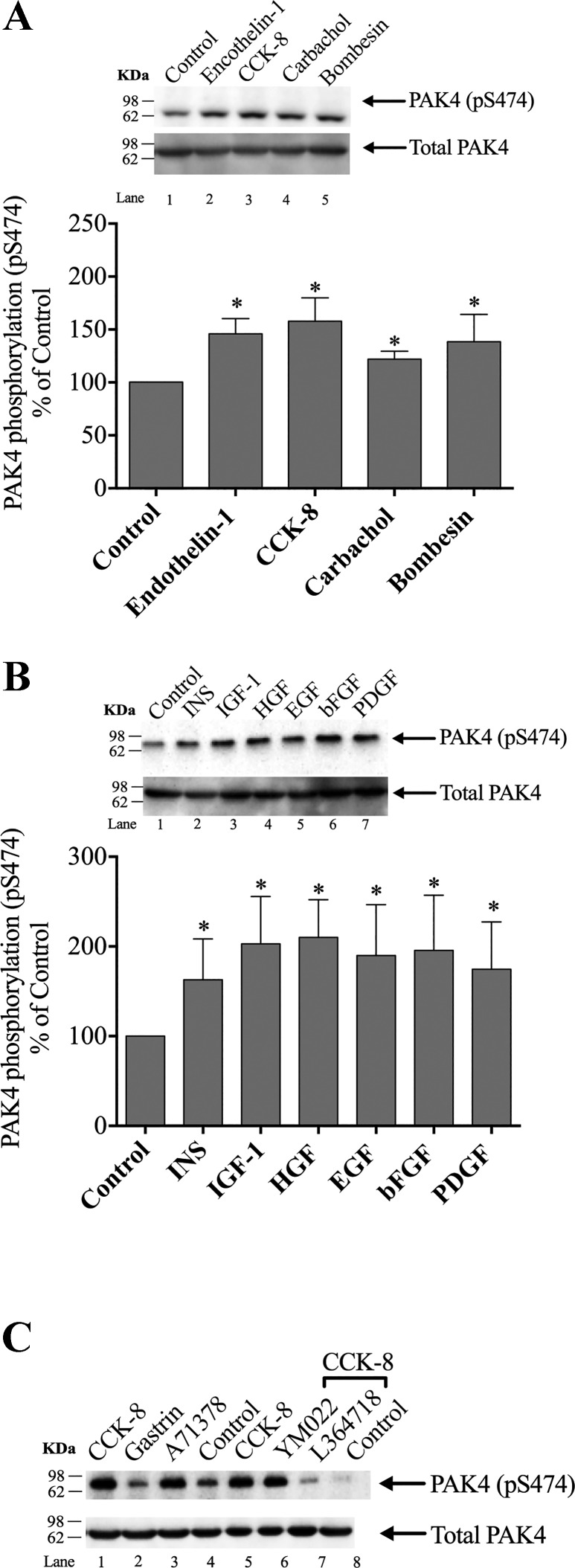
To determine if PAK4 was activated by various known GI hormones/neurotransmitters (33), rat pancreatic acini were incubated with and without CCK-8, endothelin (ET)-1, carbachol, and bombesin. As a measurement of PAK4 activation, we analyzed the phosphorylation of pS474, which has been shown to be essential for PAK4 protein kinase activity, as well as reflecting its degree of activation, and has been widely used to assess its activation in other studies (21, 64). Each hormone/neurotransmitter that activates phospholipase C (CCK-8, carbachol, and bombesin) stimulated an increase in PAK4 phosphorylation in S474 (pS474) (Fig. 2A, Lanes 3–5; Table 1). ET-1, which interacts with ET-1 and ET-3 receptors on the pancreatic acinar cell but does not activate PLC cascades or activate adenylate cyclase (29), also produced a significant increment in S474 PAK4 phosphorylation (Fig. 2A, Lane 2).
Fig. 2.
Ability of various pancreatic hormones/neurotransmitters and pancreatic growth factors to stimulate p21-activated kinase (PAK)4 phosphorylation (pS474) in rat pancreatic acini. A: ability of cholecystokinin (CCK)-8, carbachol, bombesin, or endothelin-1 to activate pS474 PAK4 in isolated pancreatic acini. Isolated pancreatic acini were incubated in the absence or presence of CCK-8 (1 nM), carbachol (10 µM), bombesin (1 nM), or endothelin-1 (10 nM) for 1 min and then lysed. Results are expressed as %control phosphorylation. B: ability of insulin (INS), insulin-like growth factor 1 (IGF-1), hepatocyte growth factor (HGF), epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), and platelet-derived growth factor (PDGF) to activate PAK4 in the pancreatic acini. Isolated pancreatic acini were incubated in the absence or presence of insulin (INS) (1 µM, 10 min), EGF (10 nM, 5 min), PDGF (100 ng/ml, 10 min), bFGF (100 ng/ml, 5 min), IGF (100 nM, 10 min), and HGF (1 nM, 10 min), and then lysed. Results expressed in A. C: representative Western blot assessing the ability of selective CCK1 or CCK2 receptor agonist/antagonists to alter PAK4 activation in pancreatic acini. Isolated pancreatic acini were incubated in the absence or presence of CCK-8 (1 nM); the CCK2 agonist, gastrin (1 nM); or the CCK1 receptor agonist, A71378 (30 nM) for 1 min; or preincubated for 5 min in the presence of the CCK2 antagonist, YM022 (1 µM); the CCK1 antagonist, L-364,718(1 µM); and then after the additional presence of CCK-8 (1 nM) for 3 min. The cell lysates were subjected to Western blotting and analyzed using anti-pS474 PAK4 and, as loading control, anti-total PAK4. Bands were visualized using chemiluminescence and quantified by densitometry. A and B, top: results of a representative blot of six independent experiments. Bottom of A and B: mean ± SE of six independent experiments. *P < 0.05 compared with the control.
Table 1.
S474 PAK4 kinase phosphorylation: characteristics and interactions of PAK4 in rat pancreatic acini
| Variable | pS474 PAK4 Phosphorylation | Concentrations and Incubation Times Reported In: |
| Stimulation by pancreatic secretagogues | Fig. 2 | |
| Yes | Endothelin-1, CCK-8, carbachol, bombesin | |
| Stimulation by pancreatic growth factors | Fig. 2 | |
| Yes | Insulin, IGF-1, HGF, EGF, bFGF, PDGF | |
| Stimulation by post-receptor activators | Fig. 5 | |
| Yes | TPA, thapsigargin, A23187 | |
| Potency/Time course | Fig. 3 | |
| EC50 of CCK-8 (nM) | 0.052 ± 0.003 | Fig. 3 |
| EC50 of CCK-JMV (nM) | 0.10 ± 0.01 | Fig. 3 |
| Inhibition of CCK-8-stimulated PAK4 activation | ||
| Inhibition of 0.3 nM CCK-8 | Fig. 7 | |
| Yes | p38 Inhibitor (SB202190), SFK inhibitor (PP2) | |
| No | p44/42 inhibitor (PD98059) | |
| Inhibition of 1 nM CCK-8 | Figs. 5–7 | |
| Yes | PKD inhibitor (CRT0066101, kbNB142–70), PKC (GFX109203) | |
| No | PI3K inhibitor (wortmannin, LY294002), Ca2+ (Thapsigargin) | |
| Inhibition of 100 nM CCK-8 | Fig. 7 | |
| Yes | p44/42 Inhibitor (PD98059) | |
| No | p38 Inhibitor (SB202190), SFK Inhibitor (PP2) | |
| Inhibition by PF-3758309 | Fig. 8 | |
| PAK4 | From 0.1 nM (IC50: 1.89 ± 0.02 nM) | |
| PAK2 | Requires high doses (i.e., ≥1 µM of PF-37580309) | |
| Inhibition by LCH-7749944 | Fig. 9 | |
| PAK4 | 90% decrease at 3 s incubation with 30 µM LCH-7749944 | |
| PAK2 | No inhibition under these conditions |
Results are calculated from data shown in Figs. 2, 3, 5–9. bFGF, basic fibroblast growth factor; CCK, cholecystokinin; EGF, epidermal growth factor; HGF, hepatocyte growth factor; IGF-1, insulin-like growth factor 1; PAK, p21-activated kinase; PDGF, platelet-derived growth factor; SFK, Src family of kinases.
All the known six pancreatic growth factors (33) tested in this study stimulated PAK4 S474 phosphorylation (Fig. 2B, Lanes 2–7; Table 1).
To determine if the CCK-8 stimulating effect on pS474 PAK4 phosphorylation was a result of the presence/activation of CCK1 or CCK2 receptors, acini cell suspension was incubated with either CCK-8, gastrin, or specific CCK1 receptor agonist, A71378 (55) (Fig. 2C, Lanes 1–3). Gastrin did not produce any increase in pS474 PAK4 phosphorylation, and the CCK-8 activation of PAK4 was mimicked by the incubation of the cells with the selective CCK1 receptor agonist, A71378. Moreover, the CCK1 receptor antagonist, L364718, but not the CCK2 receptor antagonist YM022 (7, 31), inhibited CCK-8 stimulated pS474 PAK4 phosphorylation Fig. 2C, Lanes 6–7). These results demonstrate that the effect of CCK-8 in pS474 PAK4 phosphorylation is only due to the activation of CCK1 receptors.
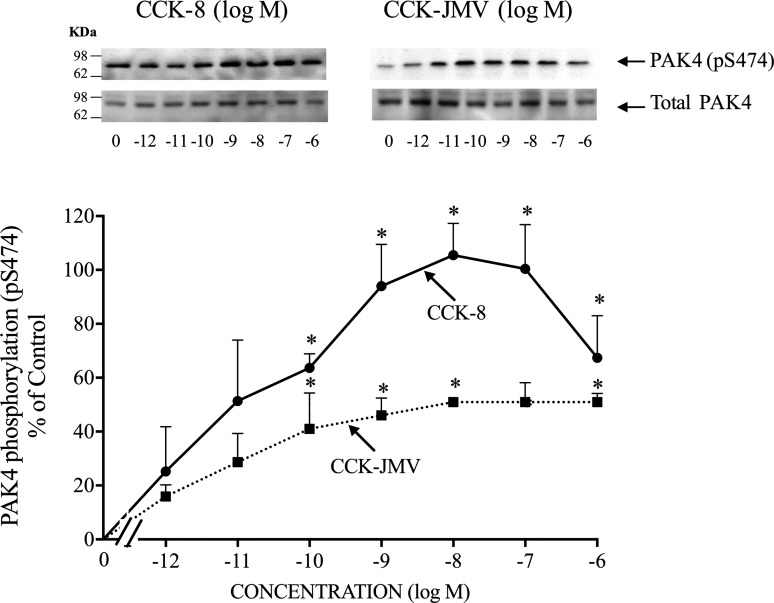
Dose-response effect on S474 PAK4 phosphorylation by CCK-8 and CCK-JMV in rat pancreatic acini.
Because CCK-8 has been shown to participate in both the normal physiology and the pathophysiology of the pancreas (13, 20, 33) and to activate the Group-I PAK, PAK2 (49, 51), we studied in detail the ability of this hormone to activate PAK4 in rat pancreatic acini. CCK-8 produced a detectible increase in S474 phosphorylation of PAK4 at 0.001 nM (Fig. 3), maximal stimulation at 1 nM CCK-8 (170 ± 10% of control), and a half-maximal effect (EC50) at 0.052 ± 0.003 nM (Fig. 3; Table 1). In pancreatic acini, the CCK1 receptor exists in two different activation states, a low and a high affinity state, which can activate different cell signaling cascades (6, 59, 68). To determine the participation of each affinity state in the activation of PAK4 by CCK-8, acini were incubated with CCK-JMV, an agonist of the CCK1 high affinity state and an antagonist of the low affinity CCK1 receptor state (6, 8, 63, 68). CCK-JMV stimulated S474 phosphorylation of PAK4 in a monophasic manner with concentrations from 0.1 nM to 1,000 nM (Fig. 3) with an EC50 of 0.10 ± 0.01 nM (Fig. 3; Table 1), and therefore was two times less potent than CCK-8. CCK-JMV caused 60% of the maximal stimulation of pS474 PAK4 phosphorylation caused by CCK-8 (Fig. 3). These results demonstrate that 60% of the CCK-8 stimulation of PAK4 activation is mediated by the high-affinity state CCK1 receptor and 40% by activation of the low affinity CCK1 receptor state.
Fig. 3.
Dose-response effect of cholecystokinin (CCK)-8 and CCK-JMV to stimulate p21-activated kinase (PAK)4 phosphorylation in rat pancreatic acini. Isolated pancreatic acini were incubated in the absence or presence of CCK-8 and CCK-JMV (at the indicate concentrations) for 3 min and then lysed. Western blots were analyzed using anti-pS474 PAK4 antibody and, as loading control, anti-total PAK4. Top: results of a representative blot of three independent experiments. Bottom: means ± SE of three independent experiments. Results are expressed as % of basal stimulation of the control group (CCK-8 1 nM: 193 ± 15% of control). *P < 0.05 compared with the control group.
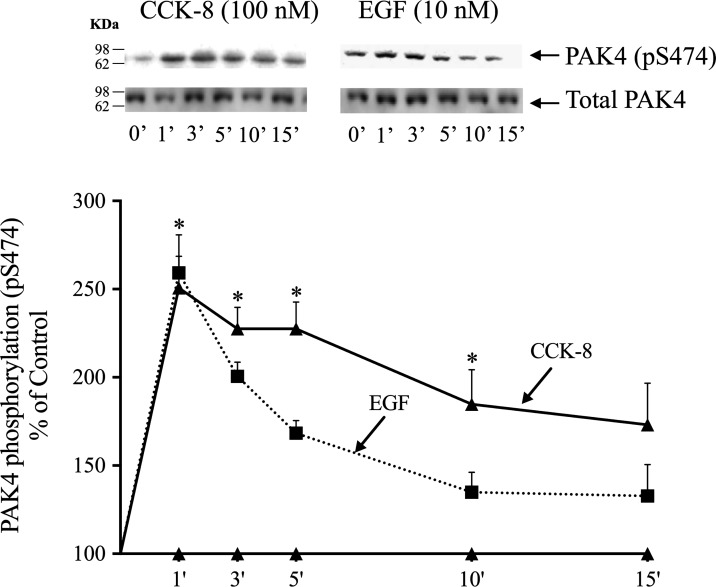
Time course of CCK-8 and EGF stimulation of S474 PAK4 phosphorylation in rat pancreatic acini.
Phosphorylation of S474 PAK4 by CCK-8 was time-dependent, with a maximum increment after 1–5-min incubation time (1–5 min: 251%–228% of control, P < 0.05 vs. control) (Fig. 4), then decreasing with further time, but still present at 10 min (Fig. 4).
Fig. 4.
Time course of cholecystokinin (CCK)-8 and epidermal growth factor (EGF) stimulation of p21-activated kinase (PAK)4 S474 phosphorylation in rat pancreatic acini. Isolated pancreatic acini were incubated in the absence or presence of CCK-8 (100 nM) or EGF (10 nM) for the indicated times, and then lysed. Western blots were analyzed using anti-pS474 PAK4 and, as loading control, anti-total PAK4. Bands were visualized using chemiluminescence and quantified by densitometry. Top: results of a representative blot of three independent experiments. Bottom: means ± SE of three independent experiments. Results are expressed as %basal stimulation of the control group. *P < 0.05 compared with the control group (i.e., 0 time).
Because the growth factors studied stimulated S474 PAK4 phosphorylation, the ability of EGF to stimulate PAK4 S474 phosphorylation at different times was studied (Fig. 4). EGF produced a rapid and maximum increase (1 min: 259 ± 22% of control, P < 0.05 vs. control) in PAK4 pS474 phosphorylation (Fig. 4), but it was not maintained after 3 min. EGF stimulation of pS474 PAK4 phosphorylation decreased over time, more rapidly than seen with CCK-8 (Fig. 4).
Effect of postreceptor stimulants, PKC inhibition, or Ca2+ depletion in CCK-8, and TPA-mediated stimulation of S474 PAK4.
Activation of PAK4 by the PKC activating agent, TPA, produced a significant increment in S474 phosphorylation of PAK4. In a calcium-containing media, the cellular calcium-mobilizing agent, TG, and the calcium ionophore, A23187, increased PAK4 phosphorylation. The combination of the calcium mobilization agent, TG, or the calcium ionophore, A23187, with the PKC activator, TPA, did not cause a greater activation than the addition of these two agents alone (Fig. 5A, Lanes 2–7).
Fig. 5.
Effect of postreceptor stimulants, PKC inhibition, or Ca2+ depletion on pS474 p21-activated kinase (PAK)4 phosphorylation by cholecystokinin (CCK)-8 and 12-O-tetradecanoylphobol-13-acetate (TPA). A: effect of the calcium ionophore, A23187 (1 μM), TPA (1 μM), thapsigargin (TG; 1 μM), or CCK-8 (1 nM) on PAK4 phosphorylation (all for 5 min, except CCK-8 for 3 min, in calcium containing media). Top: results of a representative blot of six independent experiments. Bottom: means ± SE of six independent experiments. Results are expressed as % of basal stimulation of the control group. *P < 0.05 compared with th0e control group. B: effect of PKC inhibition or Ca2+ depletion on pS474 PAK4 CCK-8- and TPA-induced phosphorylation. Isolated acini were preincubated for 2 h, alone or in the additional presence of GF109203X (GFX; 5 μM, 1 h), TG (1 μM, 1 h), or both (in a media with or without 5 mM calcium), and then incubated with or without 1 nM CCK-8 or 1 μM TPA for 3 or 5 min, respectively. Top: results of a representative blot of six independent experiments. Bottom: means ± SE of six independent experiments. Results are expressed as % of basal stimulation of the control group. *P < 0.05 compared with its control; >P < 0.05 vs. compared with similar stimulants with no additions (i.e., Ca2+ media).
We next examined whether CCK-8-induced activation of PKC or increase in intracellular calcium alone or together were needed for its ability to cause pS474 phosphorylation of PAK4 in pancreatic acini (Fig. 5B, Lanes 1–12). To study the effect of intracellular calcium changes ([Ca2+]i), pancreatic acinar cells were pretreated for 1 h in a calcium-free medium with TG (10 μM), an agent that specifically inhibits the endoplasmic reticulum Ca2+-ATPase and depletes calcium from intracellular compartments in a calcium-free medium (70). In previous studies, these conditions are reported to completely inhibit CCK-8 stimulation of [Ca2+]i in rat pancreatic acinar cells (70). To determine the participation of PKC in the CCK-8 stimulated S474 PAK4 phosphorylation, isolated acinar cells were pretreated for 1 h with a PKC inhibitor, GFX109203X (5 µM), a concentration that is known to inhibit PKC activity in these cells (70, 72).
When PKC’s activation was inhibited, TPA and CCK-8’s stimulation of serine phosphorylation of PAK4 was significantly reduced (Fig. 5B, Lanes 1–6). Inhibition of CCK-8-stimulated increases in intracellular Ca2 + did not significantly decrease the pS474 serine phosphorylation of PAK4 by CCK-8 (Fig. 5B, Lanes 7–9). The combination of both GFX and TG had an additive effect in the inhibition of basal S474 PAK4 phosphorylation and also inhibited the phosphorylation induced by CCK-8. TG significantly decreased S474 TPA stimulated PAK4 phosphorylation (Fig. 5B, Lanes 7–9). This inhibition was even higher with the combination of GFX and TG (Fig. 5B, Lane 12).
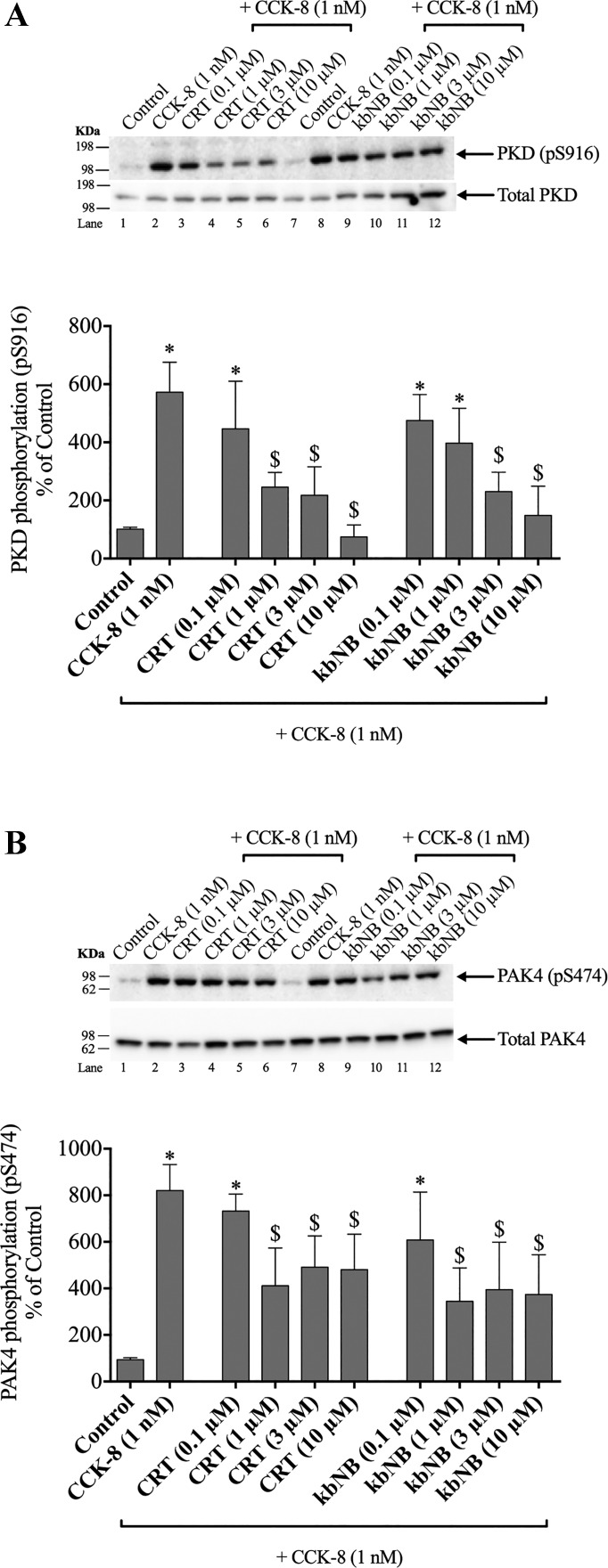
Effects of CRT0066101 and kbNB142-70, PKD inhibitors in PAK4, and PKD phosphorylation.
Previous studies (6, 70, 72) demonstrate that CCK-8 can activate PKD in both a PKC-dependent and -independent manner. To assess the effect of CCK-stimulated PKD activation on pS474 PAK4, we preincubated pancreatic acini with PKD inhibitors [CRT0066101 or kbNB142-70 (either 0.1, 1, 3, or 10 µM)] for 2 h, conditions that have been previously shown to inhibit PKD activation (14).
Preincubation with 1, 3, and 10 µM CRT0066101 significantly reduced the CCK-8-induced phosphorylation of PKD by 60, 75, and 99% (Fig. 6A, Lanes 4–6). Preincubation with 3 and 10 µM kbNB142-70 significantly reduced the CCK-8-induced phosphorylation of PKD by 72 and 90% (Fig. 6A, Lanes 10–12).
Fig. 6.
Effect of increasing concentrations of two protein kinase D (PKD) inhibitors, CRT0066101 and kbNB142-70, on PKD phosphorylation (A) and on p21-activated kinase (PAK)4 activation (B) by cholecystokinin (CCK)-8 in rat pancreatic acini. Isolated pancreatic acini were incubated in the absence or presence of CRT0066101 (CRT) or kbNB142-70 (kbNb) (0.1, 1, 3, and 10 µM) for 2 h and then incubated with no addition (control) or CCK-8 (1 nM) for 3 min, and then lysed. Western blots were analyzed using anti-pS474 PAK4 or pS916 PKD and, as loading control, anti-total PAK4 or anti-total PKD. Bands were visualized using chemiluminescence and quantified by densitometry. Top of A and B: results of a representative blot of three independent experiments. Bottom of A and B: means ± SE of at least four independent experiments. Results are expressed as % of basal stimulation of the control group. *P < 0.05 compared with the control group; $P < 0.05 compared with CCK-8 alone.
Inhibition with 1 µM CRT0066101, but not 0.1 µM, significantly inhibited CCK-8-stimulated PAK4 phosphorylation by 57 ± 11%, and the same degree of inhibition was seen with 3 and 10 µM CRT0066101 (Fig. 6B, Lanes 4–6). Incubation with kbNB142-70 also significantly decreased the pS474 serine phosphorylation of PAK4 by 60–66 ± 17%; however, as with CRT0066101, the inhibition was only partial (Fig. 6B, Lanes 10–12). These results demonstrate that under the experimental conditions used, the highest concentrations of the PKD inhibitor, CRT0066101, completely inhibited CCK-8-stimulated PKD activation; however, CCK-8 stimulated PAK4 phosphorylation was only inhibited by 60%, demonstrating that it is both PKD-dependent and PKD-independent.
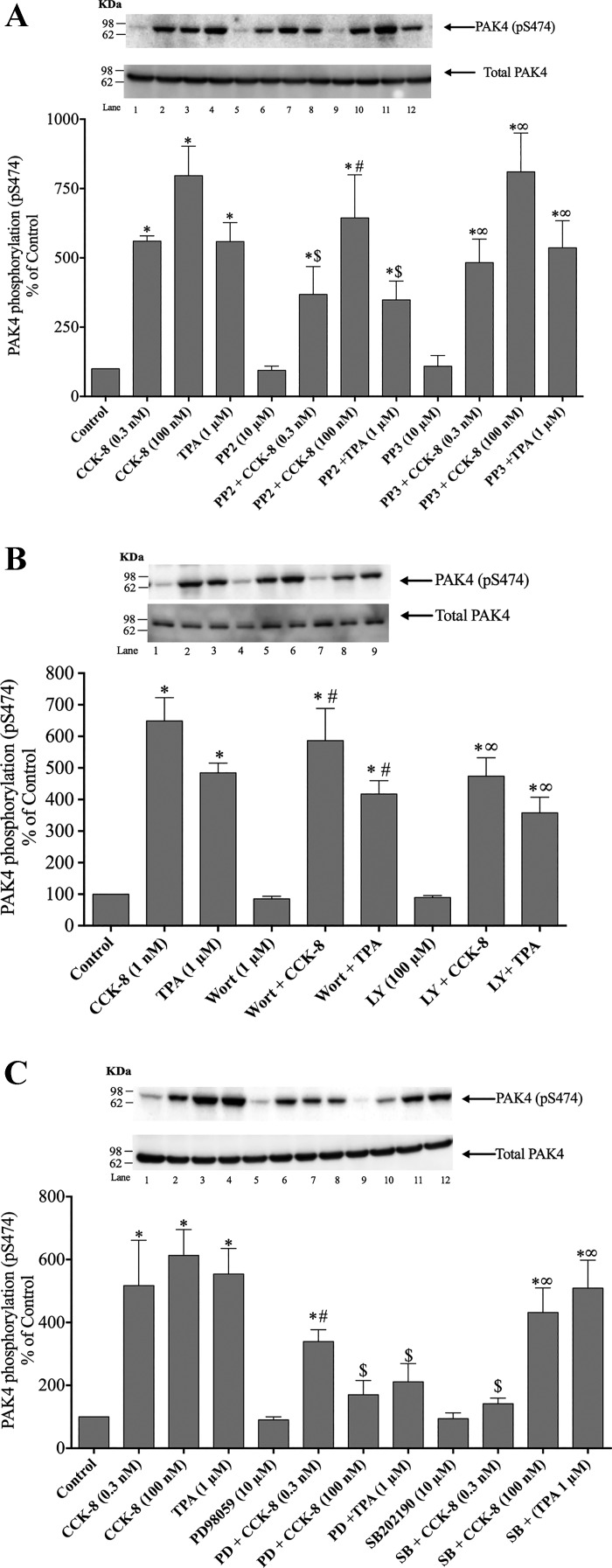
Role of Src family of kinases, phosphatidylinositol 3-kinase, p44/42, or p38 activation in mediating PAK4 phosphorylation.
In addition to activating PKC in pancreatic acini, CCK-8 and TPA also activate the Src family of kinases (SFK), phosphatidylinositol 3-kinase (PI3K), p44/42, and p38; therefore, we determined whether CCK-8- or TPA-induced PAK4 activation was dependent on stimulation of the each of these signaling molecules (8, 49, 50) (Fig. 7, A–C). To determine their possible involvement, isolated acinar cells were treated before incubation with CCK-8 (3 min) or TPA (5 min) with either the SFK specific inhibitor, PP2; with the PI3K/Akt inhibitors, Wortmannin or LY294002; or with the p44/42 or p38 inhibitors, PD98059 or SB202190, respectively, under experimental conditions that these have been shown to inhibit these signaling cascades in acini (16, 40, 49, 52, 62). The inactive analog for PP2, PP3 (10 μM), was also used to ensure specificity (8, 49, 50).
Fig. 7.
Effect of the inhibition of Src, phosphatidylinositol 3-kinase (PI3K), p44/42, or p38 MAPKs on the ability of cholecystokinin (CCK)-8 (1 nM) or 12-O-tetradecanoylphorbol-13-acetate (TPA) (1 µM) to stimulate p21-activated kinase (PAK)4. A representative experiment of at least four others and the means of all the experiments are shown. A: rat pancreatic acinar cells were pretreated with no additions or with PP2 (10 µM) or PP3 (10 µM) for 1 h and then incubated with no additions (control), 0.3 nM or 100 nM CCK-8 for 3 min or with 1 µM TPA for 5 min and then lysed and analyzed with anti-pS474 PAK4 and, as a loading control, anti-total PAK4. *P < 0.05 compared with control, #P < 0.05 vs. PP2 alone, ∞P < 0.05 vs. PP3 alone, and $P < 0.05 vs. comparing stimulants (CCK-8 or TPA) vs. stimulants preincubated with PP2. B: rat pancreatic acinar cells were pretreated with no additions or with Wortmannin (Wort; 1 μM) or LY294002 (LY; 100 μM) for 30 min and then incubated with no additions (control), 1 nM CCK-8 for 3 min, or with 1 μM TPA for 5 min and then lysed and analyzed with anti- pS474 PAK4 and, as a loading control, anti-total PAK4. *P < 0.05 compared with control, #P < 0.05 vs. CCK-8 alone, and ∞P < 0.05 vs. TPA alone. C: rat pancreatic acinar cells were pretreated with no additions or with PD98059 (PD; 10 µM) or SB202190 (SB; 10 µM) for 1 h and then incubated with no additions (control), 0.3 nM or 100 nM CCK-8 for 3 min, or with 1 µM TPA for 5 min and then lysed and analyzed with anti-pS474 PAK4 and, as a loading control, anti-total PAK4. *P < 0.05 compared with control, #P < 0.05 vs. PD98059 alone, ∞P < 0.05 vs. SB202190 alone, and $P < 0.05 vs. comparing stimulants (CCK-8 or TPA) vs. stimulants preincubated with PD98059 or SB202190.
To assess the effects of Src-inhibition on pS474 phosphorylation, we preincubated pancreatic acini with either 10 µM PP2 or 10 µM PP3 for 1 h with both a physiological (0.3 nM) and supramaximal concentration of CCK-8 (100 nM) because studies have shown they can differ in their signaling cascades (49). Inhibition of SFK did not significantly decrease either the basal pS474 phosphorylation of PAK4 or 100 nM CCK-8-stimulated PAK4 phosphorylation (Fig. 7A, Lanes 1–8). However, preincubation with PP2 decreased 0.3 nM CCK-8- and TPA-stimulated phosphorylation (Fig. 7A, Lanes 1–8). The inactive control, PP3, did not have any effect on PAK4 phosphorylation or on the positive control (Fig. 7A, Lanes 9–12).
To assess the effect of PI3K inhibition on pS474 phosphorylation, we preincubated pancreatic acini with either 1 µM Wortmannin or 100 µM LY294001 for 30 min, PI3K inhibitors that have been previously shown to be effective at inhibiting PI3K at these concentrations in pancreatic acini (8, 49, 69). When PI3K activation was inhibited with Wortmannin, the basal pS474 phosphorylation of PAK4 was not affected (Fig. 7B, Lanes 4–6). Furthermore, the increment in the serine phosphorylation of PAK4 was not significantly reduced in the case of CCK-8 or TPA (Fig. 7B, Lanes 4–6). Preincubation with 100 μM LY294002 confirmed the results obtained with Wortmannin (Fig. 7B, Lanes 7–9).
To assess the possible effect of p44/42 or p38 inhibition on pS474 phosphorylation, we preincubated pancreatic acini for 1 h with either 10 µM PD98059 or 10 µM SB202190, respectively. When p44/42 activation was inhibited, the increment in the serine phosphorylation of PAK4 was significantly reduced by 86 ± 32% in the case of CCK-8 100 nM and 76 ± 39% with TPA (Fig. 7C, Lanes 7–8). Inhibition of 0.3 nM CCK-8-stimulated p44/42 activation did not significantly decrease the pS474 serine phosphorylation of PAK4 (Fig. 7C, Lane 6). However, preincubation with the p38 inhibitor, SB202190, decreased only the 0.3 nM CCK-8-stimulated phosphorylation (Fig. 7C, Lane 10). However, SB202190 did not have any effect on 100 nM CCK-8- or TPA-stimulated PAK4 phosphorylation (Fig. 7C, Lanes 11–12).
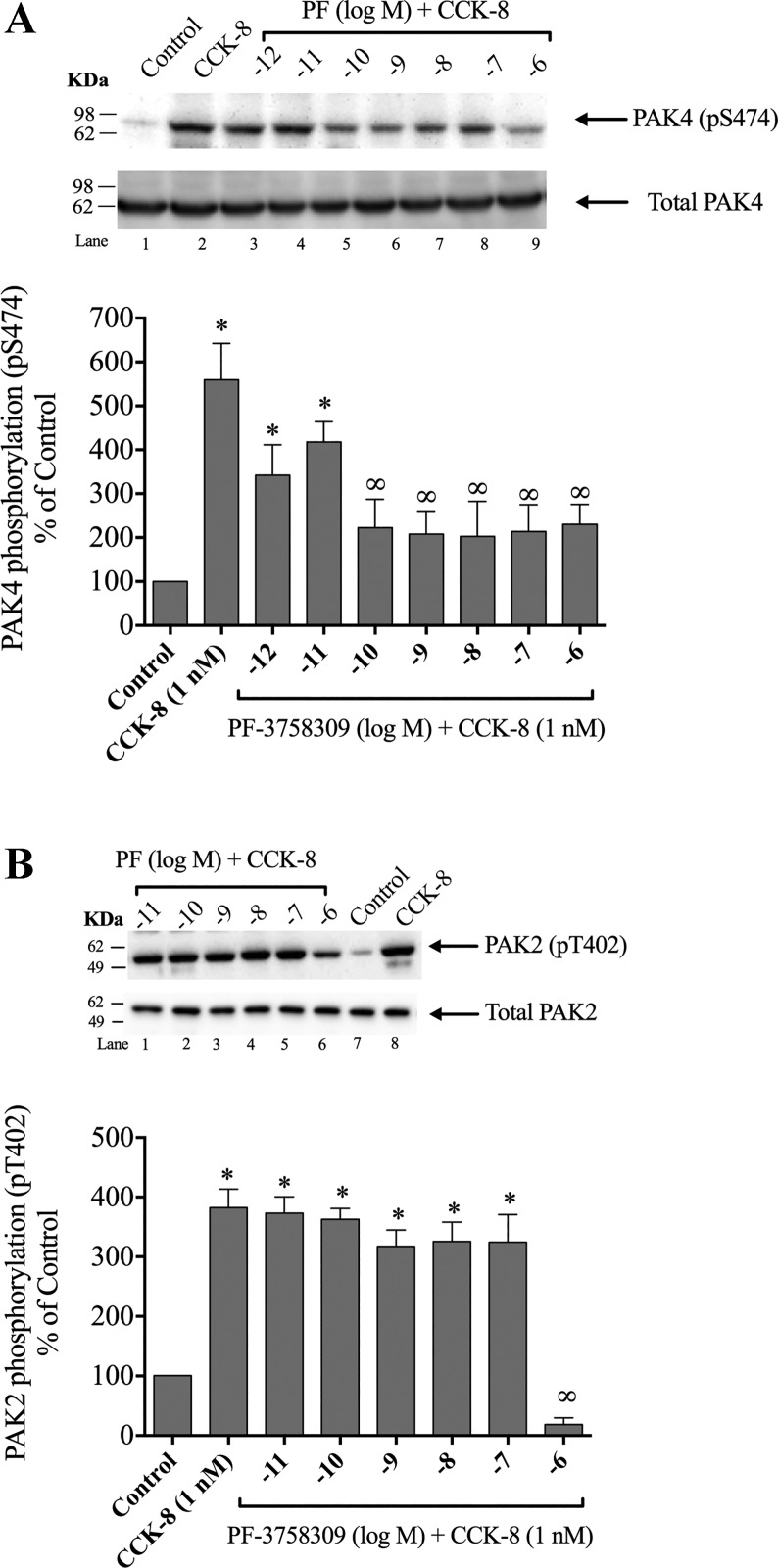
Effects of PF-3758309 and LCH-7749944 inhibitors in PAK4 and PAK2 phosphorylation.
PF-3758309 has been developed as a potent inhibitor of PAK4 and has been used in a number of studies to determine the role of PAK4 activation in various processes (48, 60). However, PF-3758309 can bind to Group-I PAKs with lower potency and at higher concentrations (48, 60). To determine the optimal experimental conditions necessary with PF-3758309 to inhibit PAK4 activation, but not PAK2, we treated the cells with increasing concentrations of PF-3758309 during a 1 h preincubation (Fig. 8A–B), then incubated the cells with 1 nM CCK-8 for 3 min (Fig. 8A–B). A 0.001-nM and 0.01-nM PF-3758309 concentration was not sufficient to induce a significant reduction of CCK-8-stimulated PAK4 activation (Fig. 8A, Lanes 3–4). However, preincubation with 0.1 nM PF-3758309 inhibited activation of PAK4 by CCK-8 (1 nM). Higher concentrations of PF-3758309 caused a similar extent of inhibition of activation of S474 phosphorylation PAK4 by CCK-8. Specifically, 0.1 nM PF-3758309 inhibited 1 nM CCK-8-stimulated PAK4 activation by 73%, and this inhibition was maintained with concentration of PF-3758309 up to 10 nM (Fig. 8A, Lanes 5–7). Because higher doses of PF-3758302 are reported in the same studies that also inhibit Group-I PAK (PAK1, 2, and 3) (48, 60), we assessed its ability to alter Group-I PAK activation in pancreatic acini. Only 1 µM PF-3758309, a 10,000-fold higher concentration than the minimal dose inhibiting PAK4 activation, produced an inhibition in PAK2 (Fig. 8B). As higher doses of PF-3758309 could lead to an inhibition of PAK2 (i.e., 1 µM PF-37580309), in our studies the 0.1-nM PF-3758309 concentration dose was used, which only inhibited PAK4 activation.
Fig. 8.
Ability of the p21-activated kinase (PAK) inhibitor II, PF-3758309 (PF), to inhibit cholecystokinin (CCK)-8 activation of PAK4 (A) or PAK2 (B). Isolated pancreatic acini were preincubated for 1 h in the presence or absence of PF-3758309 (at the indicated concentrations) and then treated with CCK-8 (1 nM) for 3 min and then lysed. Western blots were analyzed using anti-pS474 PAK4 or pT402 PAK2 antibody and, as loading control, anti-total PAK4 or anti-total PAK2. Top of A and B: results of a representative blot of three independent experiments. Bottom of A and B: means ± SE for at least five independent experiments. Results are expressed as % of basal stimulation of the control group. *P < 0.05 compared with the control group and ∞P < 0.05 compared with CCK-8 alone.
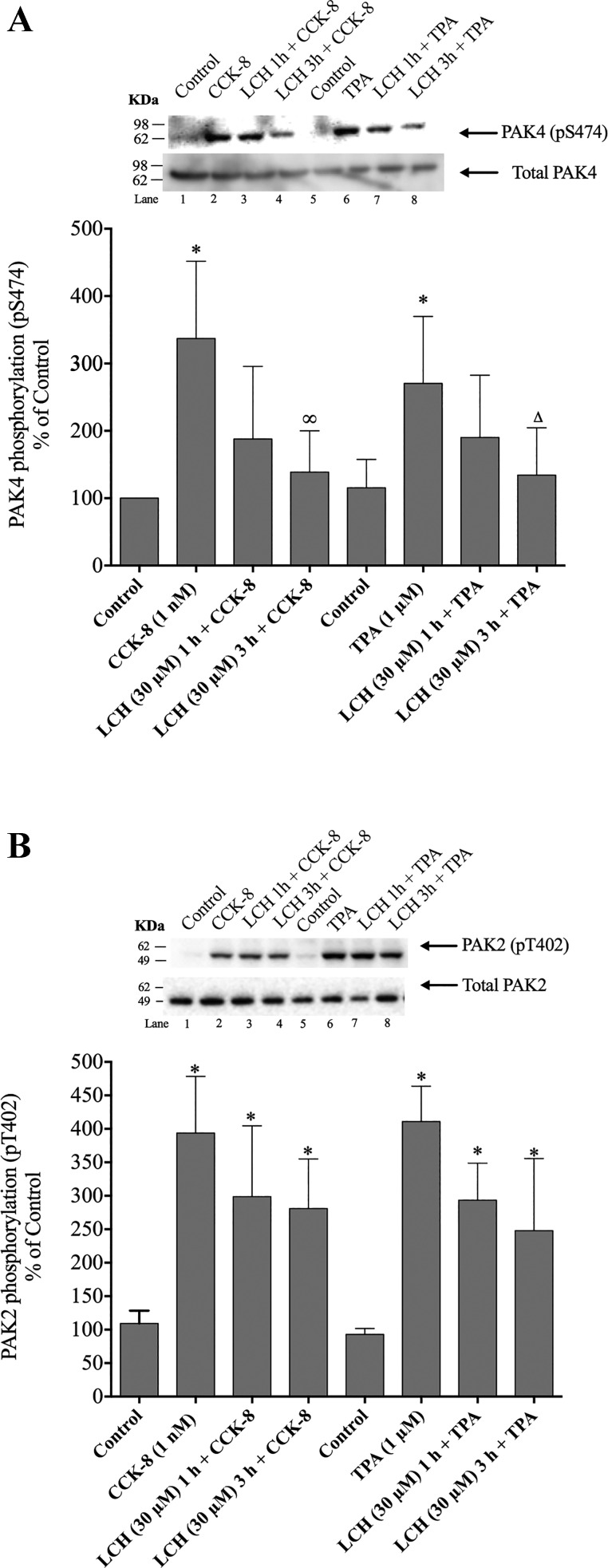
To confirm the results obtained with the PAK4 inhibitor, PF-3758309, we also used another selective PAK4 antagonist of a different class, LCH-7749944 (87). Treatment of pancreatic acini with 30 µM LCH-7749944 resulted in a reduction of PAK4 phosphorylation induced by CCK-8 or TPA, with a maximum decrease (90%) after a 3-h incubation (Fig. 9A). However, preincubation with LCH-7749944 did not inhibit CCK-8- or TPA-stimulated PAK2 phosphorylation (Fig. 9B).
Fig. 9.
Ability of the p21-activated kinase (PAK) inhibitor II, LCH-7749944 (LCH), to inhibit cholecystokinin (CCK)-8 or 12-O-tetradecanoylphobol-13-acetate (TPA) activation of PAK4 (A) or PAK2 (B). Isolated pancreatic acini were preincubated for 1 and 3 h in the presence or absence of LCH-7749944 (at the indicated concentrations) and then treated with CCK-8 (1 nM) for 3 min and TPA (1 µM) for 5 min and then lysed. Western blots were analyzed using anti-pS474 PAK4 or pT402 PAK2 antibody and, as loading control, anti-total PAK4 or anti-total PAK2. Top of A and B: results of a representative blot of three independent experiments. Bottom of A and B: means ± SE for at least five independent experiments. Results are expressed as % of basal stimulation of the control group. *P < 0.05 compared with the control group, ∞P < 0.05 compared with CCK-8 alone, and ∆P < 0.05 compared with TPA alone.
Effects of PAK4 inhibition in p44/42 activity.
Preincubation with 0.1 nM PF-3758309 and 30 µM LCH-7749944 significantly reduced the CCK-8-induced phosphorylation of p44/42 by 51% and 58%, respectively (Fig. 10). These results demonstrate that CCK-8-mediated p44/42 activation is partially dependent on PAK4 activation. Neither of the two inhibitors had an effect on basal phosphorylation of p44/42 (Fig. 10).
Fig. 10.
Effect of the p21-activated kinase (PAK)4 inhibitors, PF-3758309 (PF) or LCH-7749944 (LCH) on the ability of a physiological concentration of cholecystokinin (CCK)-8 to activate p44/42. Rat pancreatic acinar cells were pretreated with no additions or with 0.1 nM PF-3758309 or 30 µM LCH-7749944 for 3 h and then incubated with no addition (control) or with 1 nM CCK-8 for 3 min, then lysed and analyzed using anti-pT202/Y204 p42/44 and, as a loading control, anti-total p44/42. The bands were visualized using chemiluminescence and quantification of phosphorylation was assessed using scanning densitometry. Top: results of a representative blot of three independent experiments. Bottom: means ± SE for at least four independent experiments. Results are expressed as % of basal stimulation of the control group. *P < 0.05 compared with the control group and ∞P < 0.05 compared with CCK-8 alone.
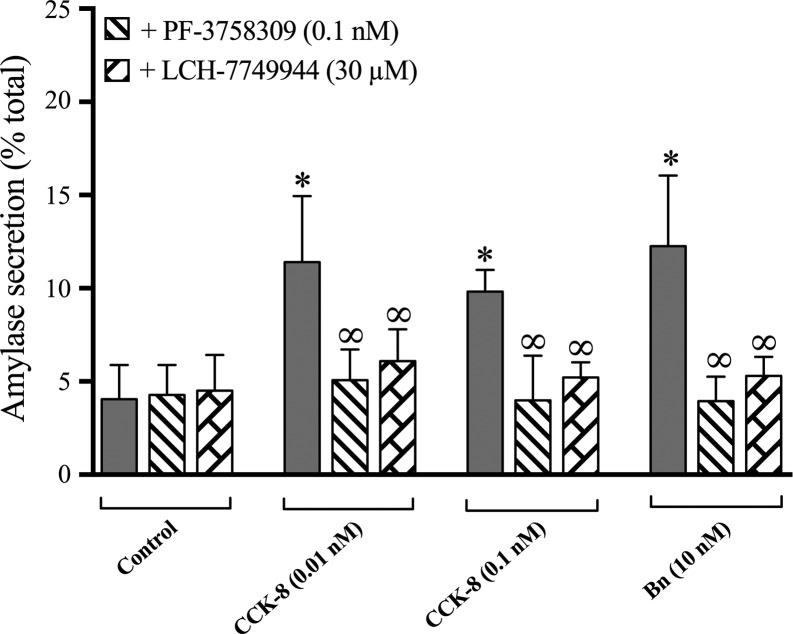
Effects of PAK4 inhibition in amylase release.
To study the possible role of PAK4 activation in enzyme secretion, acini were preincubated with either of the two PAK4 inhibitors, PF-3758309 or LCH-7749944, and treated with or without two pancreatic secretagogues [CCK-8 (0.01 nM or 0.1 nM) or bombesin (10 nM])] (Fig. 11). The two CCK-8 concentrations or bombesin alone resulted in a stimulation of enzyme secretion as previously reported in numerous studies (47, 49, 66), with 0.01 nM CCK causing a 262 ± 48% increase in amylase release, 0.1 nM CCK-8 causing a 230 ± 12% increase, and 10 nM bombesin causing an increase of 287 ± 40% (Fig. 11). Preincubation with 0.1 nM PF-3758309 inhibited 0.01 and 0.1 nM CCK-8-stimulated amylase secretion by 88 ± 10% and 95 ± 15%, respectively, as well as that induced by bombesin by 97 ± 9%. Treatment with 30 µM LCH-744944 also decreased 0.01 and 0.1 nM CCK-8-stimulated amylase secretion by 75 ± 9% and 82 ± 3%, respectively, as well as that induced by bombesin by 87 ± 8%. These results demonstrate that amylase secretion stimulated by the secretagogues is dependent on PAK4 activation. Neither of these two inhibitors had an effect on basal amylase release (Fig. 11).
Fig. 11.
Effect of the p21-activated kinase (PAK)4 inhibitors, PF-3758309 or LCH-7749944, on amylase secretion. Pancreatic acini were incubated with no additions, the indicated secretagogue alone, or with the PAK4 inhibitors PF-3758309 (0.1 nM) or LCH-7749944 (30 µM). Amylase release, expressed as percent of cellular total amylase, was determined after 30 min incubation. Results are the result of the average of six experiments. *P < 0.05 compared with the control group and ∞P < 0.05 comparing stimulants [cholecystokinin (CCK)-8 or Bombesin (Bn)] alone.
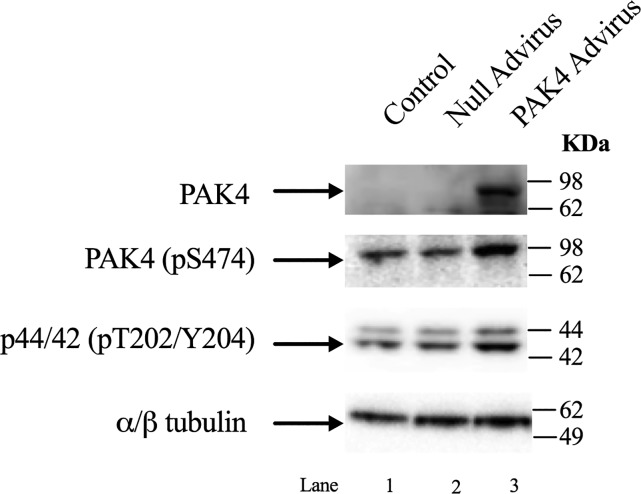
Effects of PAK4 activity modulation by preincubation with PAK4 advirus.
To corroborate the results obtained with the chemical inhibition with PF-3758309/LCH-7749944 preincubation, we infected the cells with a PAK4 Advirus that induces the cells to overexpress PAK4. Incubation without or with PAK4 Advirus resulted in the overexpression of PAK4 (Fig. 12, Row 1, Lane 3). Once the effectiveness of the virus infection was confirmed, new cells were infected with the virus as described above. After the 12 h incubation with the PAK4 Advirus, an increase in phosphorylation of 185 ± 5% and 166 ± 6% in PAK4 and p44/42, respectively, was observed (Fig. 12, Row 2–3, Lane 3). This result supports the findings obtained with the PAK4 inhibitors that PAK4 activation can affect p44/42 activity (Fig. 12). Because of the prolonged preincubation times required to infect the cells with the PAK4 Advirus construct, it was not possible to use the PAK4 Advirus to confirm the importance of PAK4 for enzyme secretion.
Fig. 12.
Effect of preincubation with p21-activated kinase (PAK)4 ad and null Adviruses upon the expression of PAK4 in pancreatic acinar cells and ERK activity. Rat pancreatic acinar cells were preincubated for 12 h without (control, Lane 1), with 109 VP/ml of null Advirus (Lane 2), and PAK4 Advirus (Lane 3). This experiment is representative of three others.
DISCUSSION
The purpose of this study was to investigate whether Group-II PAKs are expressed in pancreatic acinar cells and, if so, are activated by GI hormones/neurotransmitters/GFs. Furthermore, the signaling cascades involved with activation of PAK4 by CCK, a physiological stimulant of pancreatic acinar cell function (20), were studied, as well as the possible role of PAK4 in CCK-stimulated enzyme secretion and activation of growth cascades (20).
Whereas PAK4 has been shown to be overexpressed in many cancers, including pancreatic cancer, and plays an important role in its growth/invasiveness (64, 84), no studies have reported its expression in pancreatic acini. One study reported no expression (73), whereas another study reported PAK4 present in the pancreas, but did not state where it was in the pancreas (88). Our results demonstrate that the only Group-II PAK present in pancreatic acinar cells is PAK4. This result has similarities and differences from previous reports. Similar to our results, mRNA of the Group-II PAK, PAK5, was not detected in a study in mouse pancreas (45). However, another study showed the presence of PAKs in mouse/human pancreatic islets (5), and PAK6 is reported in pancreatic islet-like cell clusters derived from T3 cells (15). Our results, finding only PAK4 present in pancreatic acini, resemble those previously reported with pancreatic acini Group-I PAKs, in which only one PAK-I subtype (PAK2) was found (49).
Previous results demonstrate that a number of GFs (HGF, EGF, bFGF, insulin, IGF, and PDGF) are able to interact with specific receptors on pancreatic acinar cells and stimulate growth and/or protein synthesis, as well as alter other cellular functions (3, 6, 8, 52, 59, 61, 68). By assessing the specific activation of PAK4 using the phospho-specific antibody pS474 (21, 64), we observed activation of PAK4 by insulin, IGF-1, HGF, EGF, bFGF, and PDGF. Although there are no studies assessing the ability of IGF-1 or PDGF to active PAK4 in other tissues, our results have similarities with studies of the effect of the other growth factors on PAK4 activation activity in other tissues (21, 64): HGF activates PAK4 in a number of normal tissues (80) and various cancers (2, 36, 76), insulin activates PAK4 in endothelial cells (12), and EGF stimulates PAK4 activation in epithelial cells (32). In contrast to our results with PAK4, in pancreatic acini, PAK2 was not activated by insulin, IGF, or HGF, whereas similar to PAK4, PAK2 was activated by EGF, bFGF, and PDGF (49). These results demonstrate that the ability of GFs to active PAK4 varies markedly in different cells, and furthermore, can differ for PAK2 and PAK4 in the same cells.
Our results show that the GI hormones/neurotransmitters, CCK-8, bombesin, and the muscarinic receptor agonist, carbachol, which activate distinct G protein-coupled receptors on pancreatic acinar cells and stimulate PLC-mediated cascades (29, 34, 79), activate PAK4 in pancreatic acini. Also, ET-1, which interacts with specific ETA and ETB receptors on pancreatic acini (35), activated PAK4, even though it does not activate PLC cascades in these cells or stimulate secretion (35). Similarly, thyroid-stimulating hormones in papillary thyroid cancer (82), C-X-C motif chemokine 12 in prostate cancer (9), and α-MSH in B16 melanoma cells (86), each interacting with specific G protein-coupled receptors, activated PAK4. Also, similar to our results with PAK4, each of these pancreatic secretagogues activating PLC also stimulated PAK2 activation in pancreatic acini (49). In contrast to our finding with PAK4, ET-1 did not activate PAK2 in pancreatic acini (49).
In pancreatic acinar cells, CCK can stimulate numerous kinases, and their kinetics of activation can vary considerably (47, 50, 54, 62, 70). We found that CCK-8 causes a rapid activation of PAK4, which is similar to that reported for its ability to activate PAK2 and SFKs in pancreatic acini (6, 49, 62). This time course is similar to MSH activation of PAK4 in melanocytes (86); however, it differs from the activation of Group-II PAKs in human platelets by thrombin (53) or activation of PAK4 in prostate cells by the chemokine C-X-C motif chemokine 12 , which were slower (9).
The CCK1 receptor can exist in both a high- and low-affinity state, which can mediate different cellular responses (63, 70). Our results demonstrate that for maximal activation, both receptor states are required with 60% of PAK4 maximal CCK-8-stimulation because of activation of the high-affinity CCK1 receptor state and 40% to activation of the low-affinity CCK1 receptor state. This conclusion is supported by the results with CCK-8-JMV, which is a full agonist for the high-affinity CCK1 receptor state and an antagonist for the low-affinity state in rat pancreatic acini (63, 68). Our results with CCK-stimulated PAK4 activation are similar to the CCK-induced activation of PAK2 (49), Src kinases (Lyn and Yes) (52, 62), the focal adhesion kinases (p125FAK and PYK2) (70), paxillin (70), and PKD (6) in pancreatic acini, which all require activation of both low-/high- CCK1-R affinity sites for full activity. Our results with PAK4 activation differ from results with CCK-stimulated activation of PLC or PI3K, which require only high-affinity CCK1 receptor state activation (58), or activation of PKCθ or Proto-Oncogene, Adaptor Protein, CRK, which requires only low-affinity CCK1 receptor state activation (71). Our results also differ in the degree of participation of the high or low affinity state activation that is required for maximal stimulation of different cellular signaling cascades, even if they are closely related, such as PAK2 and PAK4. Specifically, this is demonstrated by the result that full activation of PAK4 requires a 2.3-fold higher (i.e., 60%) participation of the high-affinity CCK1 receptor state than PAK2 activation (26%) (49).
A number of our results support the conclusion that CCK-8 mediates PAK4 activation primarily through PKC activation and, to a lesser extent, through Ca2+ mobilization. First, the classical PKC inhibitor, GFX109203X, inhibited CCK-8-stimulated PAK4 activation, and TPA, a PKC activator, stimulated it. Second, in a calcium-containing media, the calcium ionophore, A23187, and TG, under conditions that increase cellular calcium, stimulated PAK4 activation but to a lesser extent than PKC activation. Third, TG in a calcium-free medium, which has been shown to completely inhibit CCK-8’s mobilization of cellular calcium, did not alter CCK-8-activation of PAK4, however when combined with GFX109203X, caused greater inhibition of PAK4 activation than seen with GFX109203X alone. Similar to our findings is the demonstration that formation of endothelial lumens requires PKC activation of PAK4 (38, 39), stimulated PKC is required to activate PAK4 for apical junction formation in human bronchial epithelial cells (75) and for thyroid-stimulating hormone activation of PAK4 resulting in thyroid cancer cell proliferation (82), and also are similar to the activation of PAK2 in pancreatic acini by CCK-8 (49), which is primarily mediated by PKC. In contrast to our results, Ca2+ mobilization is needed for activation of Group-II PAKs by thrombin in platelets (53) and for Group-II PAK stimulation resulting in regulation of blastomeres adhesive properties (22).
Previous results report that the PAK family can be activated by a number of different signaling cascades (4, 19, 39, 49, 64, 80); however, there is no information on the signaling cascades by which GI hormones/neurotransmitters activate Group-II PAKs, including PAK4. Our finding that CRT0066101, a PKD inhibitor, could completely inhibit CCK-8-stimulated PKD activation, but only partially inhibit CCK-8-stimulated PAK4 phosphorylation, demonstrates that CCK-8 stimulates PAK4 activation by both PKD-dependent and PKD-independent cascades. Similar to this finding, PKD activation has been shown to regulate cofilin-driven actin reorganization and cell migration through a PAK4-LIMK-cofilin mechanism (4, 19, 67).
Our results also demonstrate that activation of SFKs by physiological CCK concentrations is also an important upstream stimulator, but not activation of PI3K. This conclusion is supported by the demonstration that the SFK inhibitor, PP2, inhibited the activation of PAK4 at physiological CCK concentrations, but not at supramaximal concentrations (100 nM), suggesting that it is activation of the high affinity CCK1 receptor state that requires SFK participation for PAK4 activation. In other studies, such as endothelial cell lumen formation in endothelial cells, SFK-dependent PAK4 activation is required (39), and in vitro kinase assays demonstrate that the Src homology-3 domain can activate PAK4 (25). Our results demonstrate that PI3K did not participate in PAK4 activation, and it differs from results in gastric cancer in which activation of the PI3K/Akt-dependent pathway is required to stimulate PAK4-conferred cisplatin resistance (23) and in epithelial cells, where HGF-stimulated PI3K regulates PAK4 activity (80). However, our results are similar to CCK-activation of PAK2 in pancreatic acini, which also was not mediated by PI3K (49).
Our results also demonstrate that the activation of two MAPKs (p44/42, p38) are required for CCK-8-stimulated PAK4 activation. These findings are novel in that there is only one study (35) reporting p38 activation affecting the activity of Group-II PAKs, and no studies reporting ERK activation per se alters PAK4 activity. Except for this study (35), in all other reports ERK (46, 56, 64, 65, 78) and p38 (28, 32) activation is a downstream signaling event of PAK4 activation.
CCK stimulates pancreatic growth as well as being a physiological regulator of enzyme secretion (20, 33). PAK4 is activated by the Rho GTPases Cdc42 and, less efficiently, by Rac (26, 42, 57). Previous studies report the activation of Rac by CCK is important for pancreatic enzyme secretion and that Rho GTPases can be important mediators of growth in tissues stimulated by gastrin/CCK-related peptides (27). Activation in pancreatic acini of ERK is important in regulating growth effects (20, 30). To investigate the possible effect of PAK4 inhibition on enzyme secretion and ERK activation, we used two different classes of selective inhibitors of PAK4, PF-3758309 (48) and LCH-7749944 (87). Because some studies report that PF-3758309 can also inhibit PAK2 (48), it was first necessary to assess, under our experimental conditions, its ability to preferentially inhibit Group-II over Group-I PAK activation in pancreatic acini, to establish its specificity. Our results demonstrate under the experiment conditions used that only activation of PAK4 by CCK-8 was differentially inhibited by PF-3758309 or LCH-7749944, and not CCK-8 activation of PAK2. Our findings that PF-3758309 and LCH-7749944 show inhibition of CCK-8-stimulated enzyme secretion and ERK activation demonstrate that activation of PAK4 by CCK-8 is required for both stimulation of enzyme secretion and for stimulation of the p44/42 pathway, which has been shown to be essential for mediating CCK-8’s growth effects (20, 30). To confirm that PAK4 activation could, in fact, activate ERKs, we overexpressed PAK4 in the acinar cells using an adenovirus construct, and this resulted in PAK4 activation. Our results show that ERK inhibitors decrease PAK4 activation whereas PAK4 inhibitors decrease ERK activation and demonstrate that ERK activation is both functioning upstream of PAK4 activation as well as functioning as a downstream mediator of PAK4 activation. No previous studies report such a dual novel role of ERK in PAK4 signaling. However, a similar dual role has been reported with PI3K in gastric cancer cells (23), where PI3K activation both alters PAK4 activity and, in turn, PI3K activity is affected by PAK4 activation, demonstrating that it also functions downstream of PAK4 activation, resulting in PAK4’s and PI3K’s activation, reciprocally affecting each other’s activation (23).
We found that PAK4 is essential for CCK-8-stimulated enzyme secretion, and this may have implication for PAK-4’s role in other secretory tissues. Even though PAK4 plays an important role in cytoskeleton regulation (17, 26, 44), there are only a few other studies that have examined the role of PAK4 in secretion in any tissue. In other tissues, PAK-4 activation was found to be necessary for potentiation of insulin secretion seen with stimulation of the free fatty acid receptor in pancreatic β cells (5); in endometrial cancer, PAK4 mediates metalloprotease 4 secretion (46), and in platelets it is important in mediating degranulation and secretion (53). With the known role of Cdc42/Rac activation in mediating polarized secretion (53, 75, 85), these results, coupled with our findings, suggest that PAK4 could play an important general role in secretion in a number of tissues. Our finding that PAK4 activation stimulates ERK and is, therefore, important in mediating CCK-stimulated pancreatic growth is supported by studies in a number of tissues that demonstrate that not only does PAK4 activation frequently result in ERK activation downstream but also is important in the mediation of cell growth, cell migration, and invasion of neoplastic tissues (43, 46, 56, 65).
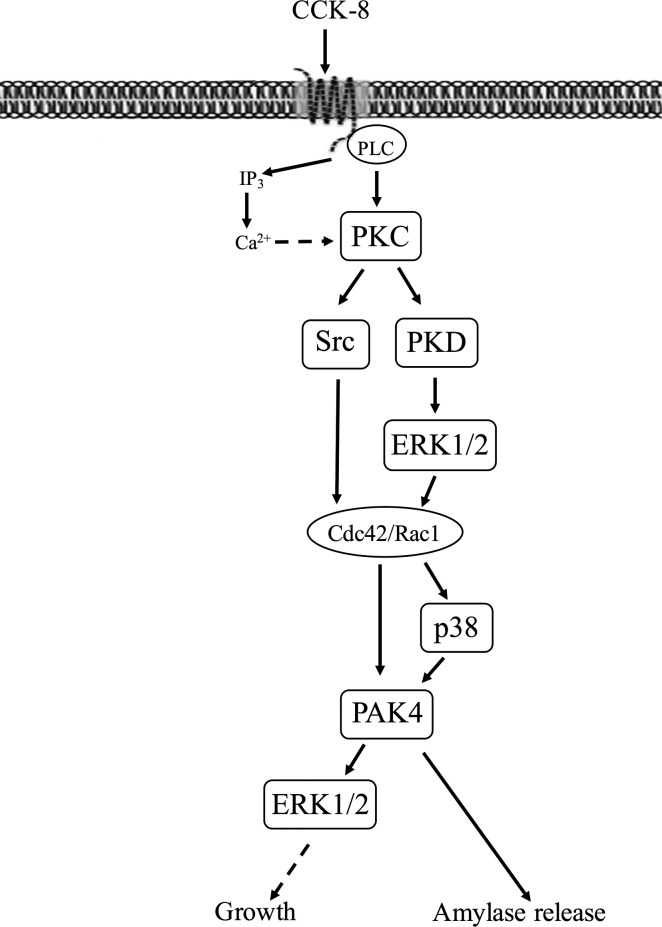
In conclusion, this study demonstrates that PAK4 is the only member of the Group-II PAKs present in pancreatic acinar cells, and it can be activated by ET, CCK-8, pancreatic GFs, and other GI hormones/neurotransmitters that activate the PLC signaling cascade. The results of our cell signaling studies are summarized diagrammatically in Fig. 13. CCK-8-stimulated PAK4 activation is mediated primarily by PKC activation and is novel in that it involves both PKD-independent cascades and PKD-dependent cascades. Furthermore, activation of SFK is required for full PAK4 activation, as well as p44/42 and p38 activation, but in contrast to PAK4’s activation in a number of other cells, PI3K activation is not required. PAK4 activation is also important in mediating p44/42 activation, which in a number of studies is needed for CCK-8-induced growth. These results are a novel finding in that p44/42 activation and PAK4 activation reciprocally affect each other’s activation in pancreatic acini. CCK-8-mediated PAK4 activation is also needed for stimulated enzyme secretion. Our results support the conclusion that activation of PAK4 in pancreatic acinar cells by GI hormones/neurotransmitters and GI GFs may be an important signaling cascade in physiological responses, similar to those shown in recent studies with PAK2 in pancreatic acini (74). Many of the stimuli that activate PAK4 in this study are also involved in pathophysiological processes affecting acini; therefore, PAK4’s role in these processes, such as pancreatitis, should be explored in the future.
Fig. 13.
Schematic diagram of signaling cascade for activation of p21-activated kinase (PAK)4 in pancreatic acinar cells. In rat pancreatic acinar cells, maximal activation of PAK4 by cholecystokinin (CCK)-8 requires activation of primarily PKC, with a lesser contribution by changes in cytosolic calcium. PKC mediates both Src family of kinase (SFK) and protein kinase D (PKD) activation with the latter resulting in ERK1/2 activation and cell division control protein 42 homolog (Cdc42)/ Ras-related C3 botulinum toxin substrate 1 (Rac1) activation. This in turn can stimulate PAK4 activation partially via a p38 mechanism. PAK4 activation is important for CCK-stimulated enzyme secretion as well as ERK1/2 activation which has been shown to mediate growth (20, 30). However, changes in phosphatidylinositol 3-kinase (PI3K) are not involved but were reported in a number of other tissues (23, 80). The ERK1/2 and PAK4 inhibition studies demonstrate CCK-8-mediated ERK1/2 activation and PAK4 activation reciprocally regulate each other’s activation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.R.-A. and R.T.J. conceived and designed research; performed experiments; analyzed data; interpreted results of experiments; prepared figures; drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
ACKNOWLEDGMENTS
This work is partially supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, NIH.
REFERENCES
- 1.Abo A, Qu J, Cammarano MS, Dan C, Fritsch A, Baud V, Belisle B, Minden A. PAK4, a novel effector for Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia. EMBO J 17: 6527–6540, 1998. doi: 10.1093/emboj/17.22.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed T, Shea K, Masters JR, Jones GE, Wells CMA. A PAK4-LIMK1 pathway drives prostate cancer cell migration downstream of HGF. Cell Signal 20: 1320–1328, 2008. doi: 10.1016/j.cellsig.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 3.Aparicio IM, Garcia-Marin LJ, Andreolotti AG, Bodega G, Jensen RT, Bragado MJ. Hepatocyte growth factor activates several transduction pathways in rat pancreatic acini. Biochim Biophys Acta 1643: 37–46, 2003. doi: 10.1016/j.bbamcr.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Bastea LI, Döppler H, Pearce SE, Durand N, Spratley SJ, Storz P. Protein kinase D-mediated phosphorylation at Ser99 regulates localization of p21-activated kinase 4. Biochem J 455: 251–260, 2013. doi: 10.1042/BJ20130281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergeron V, Ghislain J, Poitout V. The P21-activated kinase PAK4 is implicated in fatty-acid potentiation of insulin secretion downstream of free fatty acid receptor 1. Islets 8: 157–164, 2016. doi: 10.1080/19382014.2016.1243191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berna MJ, Hoffmann KM, Tapia JA, Thill M, Pace A, Mantey SA, Jensen RT. CCK causes PKD1 activation in pancreatic acini by signaling through PKC-delta and PKC-independent pathways. Biochim Biophys Acta 1773: 483–501, 2007. doi: 10.1016/j.bbamcr.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berna MJ, Tapia JA, Sancho V, Jensen RT. Progress in developing cholecystokinin (CCK)/gastrin receptor ligands that have therapeutic potential. Curr Opin Pharmacol 7: 583–592, 2007. doi: 10.1016/j.coph.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berna MJ, Tapia JA, Sancho V, Thill M, Pace A, Hoffmann KM, Gonzalez-Fernandez L, Jensen RT. Gastrointestinal growth factors and hormones have divergent effects on Akt activation. Cell Signal 21: 622–638, 2009. doi: 10.1016/j.cellsig.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhardwaj A, Srivastava SK, Singh S, Arora S, Tyagi N, Andrews J, McClellan S, Carter JE, Singh AP. CXCL12/CXCR4 signaling counteracts docetaxel-induced microtubule stabilization via p21-activated kinase 4-dependent activation of LIM domain kinase 1. Oncotarget 5: 11490–11500, 2014. doi: 10.18632/oncotarget.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bissonnette BM, Collen MJ, Adachi H, Jensen RT, Gardner JD. Receptors for vasoactive intestinal peptide and secretin on rat pancreatic acini. Am J Physiol 246: G710–G717, 1984. doi: 10.1152/ajpgi.1984.246.6.G710. [DOI] [PubMed] [Google Scholar]
- 11.Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem 72: 743–781, 2003. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 12.Bowers SL, Norden PR, Davis GE. Molecular signaling pathways controlling vascular tube morphogenesis and pericyte-induced tube maturation in 3D extracellular matrices. Adv Pharmacol 77: 241–280, 2016. doi: 10.1016/bs.apha.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Chandra R, Liddle RA. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes 14: 63–67, 2007. doi: 10.1097/MED.0b013e3280122850. [DOI] [PubMed] [Google Scholar]
- 14.Chang JK, Ni Y, Han L, Sinnett-Smith J, Jacamo R, Rey O, Young SH, Rozengurt E. Protein kinase D1 (PKD1) phosphorylation on Ser203 by type I p21-activated kinase (PAK) regulates PKD1 localization. J Biol Chem 292: 9523–9539, 2017. doi: 10.1074/jbc.M116.771394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen BZ, Yu SL, Singh S, Kao LP, Tsai ZY, Yang PC, Chen BH, Shoei-Lung Li S. Identification of microRNAs expressed highly in pancreatic islet-like cell clusters differentiated from human embryonic stem cells. Cell Biol Int 35: 29–37, 2011. doi: 10.1042/CBI20090081. [DOI] [PubMed] [Google Scholar]
- 16.Dabrowski A, Tribillo I, Dabrowska MI, Wereszczynska-Siemiatkowska U, Gabryelewicz A. Activation of mitogen-activated protein kinases in different models of pancreatic acinar cell damage. Z Gastroenterol 38: 469–481, 2000. doi: 10.1055/s-2000-14885. [DOI] [PubMed] [Google Scholar]
- 17.Dart AE, Wells CM. P21-activated kinase 4--not just one of the PAK. Eur J Cell Biol 92: 129–138, 2013. doi: 10.1016/j.ejcb.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Dechert MA, Holder JM, Gerthoffer WT. p21-activated kinase 1 participates in tracheal smooth muscle cell migration by signaling to p38 Mapk. Am J Physiol Cell Physiol 281: C123–C132, 2001. doi: 10.1152/ajpcell.2001.281.1.C123. [DOI] [PubMed] [Google Scholar]
- 19.Döppler H, Bastea LI, Borges S, Spratley SJ, Pearce SE, Storz P. Protein kinase d isoforms differentially modulate cofilin-driven directed cell migration. PLoS One 9: e98090, 2014. doi: 10.1371/journal.pone.0098090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dufresne M, Seva C, Fourmy D. Cholecystokinin and gastrin receptors. Physiol Rev 86: 805–847, 2006. doi: 10.1152/physrev.00014.2005. [DOI] [PubMed] [Google Scholar]
- 21.Eswaran J, Lee WH, Debreczeni JE, Filippakopoulos P, Turnbull A, Fedorov O, Deacon SW, Peterson JR, Knapp S. Crystal structures of the p21-activated kinases PAK4, PAK5, and PAK6 reveal catalytic domain plasticity of active group II PAKs. Structure 15: 201–213, 2007. doi: 10.1016/j.str.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faure S, Cau J, de Santa Barbara P, Bigou S, Ge Q, Delsert C, Morin N. Xenopus p21-activated kinase 5 regulates blastomeres’ adhesive properties during convergent extension movements. Dev Biol 277: 472–492, 2005. doi: 10.1016/j.ydbio.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Fu X, Feng J, Zeng D, Ding Y, Yu C, Yang B. PAK4 confers cisplatin resistance in gastric cancer cells via PI3K/Akt- and MEK/ERK-dependent pathways. Biosci Rep 34: e00094, 2014. doi: 10.1042/BSR20130102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García LJ, Rosado JA, González A, Jensen RT. Cholecystokinin-stimulated tyrosine phosphorylation of p125FAK and paxillin is mediated by phospholipase C-dependent and -independent mechanisms and requires the integrity of the actin cytoskeleton and participation of p21rho. Biochem J 327: 461–472, 1997. doi: 10.1042/bj3270461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ha BH, Davis MJ, Chen C, Lou HJ, Gao J, Zhang R, Krauthammer M, Halaban R, Schlessinger J, Turk BE, Boggon TJ. Type II p21-activated kinases (PAKs) are regulated by an autoinhibitory pseudosubstrate. Proc Natl Acad Sci USA 109: 16107–16112, 2012. doi: 10.1073/pnas.1214447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ha BH, Morse EM, Turk BE, Boggon TJ. Signaling, regulation, and specificity of the type II p21-activated kinases. J Biol Chem 290: 12975–12983, 2015. doi: 10.1074/jbc.R115.650416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He H, Baldwin GS. Rho GTPases and p21-activated kinase in the regulation of proliferation and apoptosis by gastrins. Int J Biochem Cell Biol 40: 2018–2022, 2008. doi: 10.1016/j.biocel.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 28.He W, Zhao Z, Anees A, Li Y, Ashraf U, Chen Z, Song Y, Chen H, Cao S, Ye J. p21-Activated kinase 4 signaling promotes Japanese encephalitis virus-mediated inflammation in astrocytes. Front Cell Infect Microbiol 7: 271, 2017. doi: 10.3389/fcimb.2017.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hildebrand P, Mrozinski JE Jr, Mantey SA, Patto RJ, Jensen RT. Pancreatic acini possess endothelin receptors whose internalization is regulated by PLC-activating agents. Am J Physiol 264: G984–G993, 1993. doi: 10.1152/ajpgi.1993.264.5.G984. [DOI] [PubMed] [Google Scholar]
- 30.Holtz BJ, Lodewyk KB, Sebolt-Leopold JS, Ernst SA, Williams JA. ERK activation is required for CCK-mediated pancreatic adaptive growth in mice. Am J Physiol Gastrointest Liver Physiol 307: G700–G710, 2014. doi: 10.1152/ajpgi.00163.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang SC, Zhang L, Chiang HC, Wank SA, Maton PN, Gardner JD, Jensen RT. Benzodiazepine analogues L365,260 and L364,718 as gastrin and pancreatic CCK receptor antagonists. Am J Physiol 257: G169–G174, 1989. doi: 10.1152/ajpgi.1989.257.1.G169. [DOI] [PubMed] [Google Scholar]
- 32.Huang Y, Mikami F, Jono H, Zhang W, Weng X, Koga T, Xu H, Yan C, Kai H, Li JD. Opposing roles of PAK2 and PAK4 in synergistic induction of MUC5AC mucin by bacterium NTHi and EGF. Biochem Biophys Res Commun 359: 691–696, 2007. doi: 10.1016/j.bbrc.2007.05.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen RT. Receptors on pancreatic acinar cells (3rd ed.). In: Physiology of the Gastrointestinal Tract, edited by Johnson LR, Jacobson ED, Christensen J, Alpers DH, Walsh JH. New York: Raven, 1994, p. 1377–1446. [Google Scholar]
- 34.Jensen RT, Gardner JD. Identification and characterization of receptors for secretagogues on pancreatic acinar cells. Fed Proc 40: 2486–2496, 1981. [PubMed] [Google Scholar]
- 35.Kaur R, Liu X, Gjoerup O, Zhang A, Yuan X, Balk SP, Schneider MC, Lu ML. Activation of p21-activated kinase 6 by MAP kinase kinase 6 and p38 MAP kinase. J Biol Chem 280: 3323–3330, 2005. doi: 10.1074/jbc.M406701200. [DOI] [PubMed] [Google Scholar]
- 36.Kim H, Woo DJ, Kim SY, Yang EG. p21-activated kinase 4 regulates HIF-1α translation in cancer cells. Biochem Biophys Res Commun 486: 270–276, 2017. doi: 10.1016/j.bbrc.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 37.King H, Nicholas NS, Wells CM. Role of p-21-activated kinases in cancer progression. Int Rev Cell Mol Biol 309: 347–387, 2014. doi: 10.1016/B978-0-12-800255-1.00007-7. [DOI] [PubMed] [Google Scholar]
- 38.Koh W, Mahan RD, Davis GE. Cdc42- and Rac1-mediated endothelial lumen formation requires Pak2, Pak4 and Par3, and PKC-dependent signaling. J Cell Sci 121: 989–1001, 2008. doi: 10.1242/jcs.020693. [DOI] [PubMed] [Google Scholar]
- 39.Koh W, Sachidanandam K, Stratman AN, Sacharidou A, Mayo AM, Murphy EA, Cheresh DA, Davis GE. Formation of endothelial lumens requires a coordinated PKCepsilon-, Src-, Pak- and Raf-kinase-dependent signaling cascade downstream of Cdc42 activation. J Cell Sci 122: 1812–1822, 2009. doi: 10.1242/jcs.045799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kubisch CH, Sans MD, Arumugam T, Ernst SA, Williams JA, Logsdon CD. Early activation of endoplasmic reticulum stress is associated with arginine-induced acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 291: G238–G245, 2006. doi: 10.1152/ajpgi.00471.2005. [DOI] [PubMed] [Google Scholar]
- 41.Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer 6: 459–471, 2006. doi: 10.1038/nrc1892. [DOI] [PubMed] [Google Scholar]
- 42.Kumar R, Sanawar R, Li X, Li F. Structure, biochemistry, and biology of PAK kinases. Gene 605: 20–31, 2017. doi: 10.1016/j.gene.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li SQ, Wang ZH, Mi XG, Liu L, Tan Y. MiR-199a/b-3p suppresses migration and invasion of breast cancer cells by downregulating PAK4/MEK/ERK signaling pathway. IUBMB Life 67: 768–777, 2015. doi: 10.1002/iub.1433. [DOI] [PubMed] [Google Scholar]
- 44.Li X, Li J, Li F. P21 activated kinase 4 binds translation elongation factor eEF1A1 to promote gastric cancer cell migration and invasion. Oncol Rep 37: 2857–2864, 2017. doi: 10.3892/or.2017.5543. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Minden A. Targeted disruption of the gene for the PAK5 kinase in mice. Mol Cell Biol 23: 7134–7142, 2003. doi: 10.1128/MCB.23.20.7134-7142.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu W, Xia YH, Qu JJ, He YY, Li BL, Lu C, Luo X, Wan XP. p21-activated kinase 4 regulation of endometrial cancer cell migration and invasion involves the ERK1/2 pathway mediated MMP-2 secretion. Neoplasma 60: 493–503, 2013. doi: 10.4149/neo_2013_064. [DOI] [PubMed] [Google Scholar]
- 47.Lynch G, Kohler S, Leser J, Beil M, Garcia-Marin LJ, Lutz MP. The tyrosine kinase Yes regulates actin structure and secretion during pancreatic acinar cell damage in rats. Pflugers Arch 447: 445–451, 2004. doi: 10.1007/s00424-003-1188-7. [DOI] [PubMed] [Google Scholar]
- 48.Murray BW, Guo C, Piraino J, Westwick JK, Zhang C, Lamerdin J, Dagostino E, Knighton D, Loi CM, Zager M, Kraynov E, Popoff I, Christensen JG, Martinez R, Kephart SE, Marakovits J, Karlicek S, Bergqvist S, Smeal T. Small-molecule p21-activated kinase inhibitor PF-3758309 is a potent inhibitor of oncogenic signaling and tumor growth. Proc Natl Acad Sci USA 107: 9446–9451, 2010. doi: 10.1073/pnas.0911863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nuche-Berenguer B, Jensen RT. Gastrointestinal hormones/neurotransmitters and growth factors can activate P21 activated kinase 2 in pancreatic acinar cells by novel mechanisms. Biochim Biophys Acta 1853, 10 Pt A: 2371–2382, 2015. doi: 10.1016/j.bbamcr.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nuche-Berenguer B, Moreno P, Jensen RT. Elucidation of the roles of the Src kinases in pancreatic acinar cell signaling. J Cell Biochem 116: 22–36, 2015. doi: 10.1002/jcb.24895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nuche-Berenguer B, Ramos-Álvarez I, Jensen RT. The p21-activated kinase, PAK2, is important in the activation of numerous pancreatic acinar cell signaling cascades and in the onset of early pancreatitis events. Biochim Biophys Acta 1862: 1122–1136, 2016. doi: 10.1016/j.bbadis.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pace A, Tapia JA, Garcia-Marin LJ, Jensen RT. The Src family kinase, Lyn, is activated in pancreatic acinar cells by gastrointestinal hormones/neurotransmitters and growth factors which stimulate its association with numerous other signaling molecules. Biochim Biophys Acta 1763: 356–365, 2006. doi: 10.1016/j.bbamcr.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Pandey D, Goyal P, Dwivedi S, Siess W. Unraveling a novel Rac1-mediated signaling pathway that regulates cofilin dephosphorylation and secretion in thrombin-stimulated platelets. Blood 114: 415–424, 2009. doi: 10.1182/blood-2008-10-183582. [DOI] [PubMed] [Google Scholar]
- 54.Piiper A, Elez R, You SJ, Kronenberger B, Loitsch S, Roche S, Zeuzem S. Cholecystokinin stimulates extracellular signal-regulated kinase through activation of the epidermal growth factor receptor, Yes, and protein kinase C. Signal amplification at the level of Raf by activation of protein kinase Cepsilon. J Biol Chem 278: 7065–7072, 2003. doi: 10.1074/jbc.M211234200. [DOI] [PubMed] [Google Scholar]
- 55.Qian JM, Rowley WH, Jensen RT. Gastrin and CCK activate phospholipase C and stimulate pepsinogen release by interacting with two distinct receptors. Am J Physiol 264: G718–G727, 1993. doi: 10.1152/ajpgi.1993.264.4.G718. [DOI] [PubMed] [Google Scholar]
- 56.Rafn B, Nielsen CF, Andersen SH, Szyniarowski P, Corcelle-Termeau E, Valo E, Fehrenbacher N, Olsen CJ, Daugaard M, Egebjerg C, Bøttzauw T, Kohonen P, Nylandsted J, Hautaniemi S, Moreira J, Jäättelä M, Kallunki T. ErbB2-driven breast cancer cell invasion depends on a complex signaling network activating myeloid zinc finger-1-dependent cathepsin B expression. Mol Cell 45: 764–776, 2012. doi: 10.1016/j.molcel.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 57.Rane CK, Minden A. P21 activated kinases: structure, regulation, and functions. Small GTPases 5: e28003, 2014. doi: 10.4161/sgtp.28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rivard N, Rydzewska G, Lods JS, Martinez J, Morisset J. Pancreas growth, tyrosine kinase, PtdIns 3-kinase, and PLD involve high-affinity CCK-receptor occupation. Am J Physiol 266: G62–G70, 1994. doi: 10.1152/ajpgi.1994.266.1.G62. [DOI] [PubMed] [Google Scholar]
- 59.Rowley WH, Sato S, Huang SC, Collado-Escobar DM, Beaven MA, Wang LH, Martinez J, Gardner JD, Jensen RT. Cholecystokinin-induced formation of inositol phosphates in pancreatic acini. Am J Physiol 259: G655–G665, 1990. doi: 10.1152/ajpgi.1990.259.4.G655. [DOI] [PubMed] [Google Scholar]
- 60.Rudolph J, Crawford JJ, Hoeflich KP, Wang W. Inhibitors of p21-activated kinases (PAKs). J Med Chem 58: 111–129, 2015. doi: 10.1021/jm501613q. [DOI] [PubMed] [Google Scholar]
- 61.Sancho V, Berna MJ, Thill M, Jensen RT. PKCθ activation in pancreatic acinar cells by gastrointestinal hormones/neurotransmitters and growth factors is needed for stimulation of numerous important cellular signaling cascades. Biochim Biophys Acta 1813: 2145–2156, 2011. doi: 10.1016/j.bbamcr.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sancho V, Nuche-Berenguer B, Jensen RT. The Src kinase Yes is activated in pancreatic acinar cells by gastrointestinal hormones/neurotransmitters, but not pancreatic growth factors, which stimulate its association with numerous other signaling molecules. Biochim Biophys Acta 1823: 1285–1294, 2012. doi: 10.1016/j.bbamcr.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sato S, Stark HA, Martinez J, Beaven MA, Jensen RT, Gardner JD. Receptor occupation, calcium mobilization, and amylase release in pancreatic acini: effect of CCK-JMV-180. Am J Physiol 257: G202–G209, 1989. doi: 10.1152/ajpgi.1989.257.2.G202. [DOI] [PubMed] [Google Scholar]
- 64.Shao YG, Ning K, Li F. Group II p21-activated kinases as therapeutic targets in gastrointestinal cancer. World J Gastroenterol 22: 1224–1235, 2016. doi: 10.3748/wjg.v22.i3.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Siu MK, Chan HY, Kong DS, Wong ES, Wong OG, Ngan HY, Tam KF, Zhang H, Li Z, Chan QK, Tsao SW, Strömblad S, Cheung AN. p21-activated kinase 4 regulates ovarian cancer cell proliferation, migration, and invasion and contributes to poor prognosis in patients. Proc Natl Acad Sci USA 107: 18622–18627, 2010. doi: 10.1073/pnas.0907481107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spanarkel M, Martinez J, Briet C, Jensen RT, Gardner JD. Cholecystokinin-27-32-amide. A member of a new class of cholecystokinin receptor antagonists. J Biol Chem 258: 6746–6749, 1983. [PubMed] [Google Scholar]
- 67.Spratley SJ, Bastea LI, Döppler H, Mizuno K, Storz P. Protein kinase D regulates cofilin activity through p21-activated kinase 4. J Biol Chem 286: 34254–34261, 2011. doi: 10.1074/jbc.M111.259424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stark HA, Sharp CM, Sutliff VE, Martinez J, Jensen RT, Gardner JD. CCK-JMV-180: a peptide that distinguishes high-affinity cholecystokinin receptors from low-affinity cholecystokinin receptors. Biochim Biophys Acta 1010: 145–150, 1989. doi: 10.1016/0167-4889(89)90154-7. [DOI] [PubMed] [Google Scholar]
- 69.Tapia JA, Camello C, Jensen RT, Garcia LJ. EGF stimulates tyrosine phosphorylation of focal adhesion kinase (p125FAK) and paxillin in rat pancreatic acini by a phospholipase C-independent process that depends on phosphatidylinositol 3-kinase, the small GTP-binding protein, p21rho, and the integrity of the actin cytoskeleton. Biochim Biophys Acta 1448: 486–499, 1999. doi: 10.1016/S0167-4889(98)00157-8. [DOI] [PubMed] [Google Scholar]
- 70.Tapia JA, Ferris HA, Jensen RT, García LJ. Cholecystokinin activates PYK2/CAKbeta by a phospholipase C-dependent mechanism and its association with the mitogen-activated protein kinase signaling pathway in pancreatic acinar cells. J Biol Chem 274: 31261–31271, 1999. doi: 10.1074/jbc.274.44.31261. [DOI] [PubMed] [Google Scholar]
- 71.Tapia JA, García-Marin LJ, Jensen RT. Cholecystokinin-stimulated protein kinase C-delta kinase activation, tyrosine phosphorylation, and translocation are mediated by Src tyrosine kinases in pancreatic acinar cells. J Biol Chem 278: 35220–35230, 2003. doi: 10.1074/jbc.M303119200. [DOI] [PubMed] [Google Scholar]
- 72.Thrower EC, Wang J, Cheriyan S, Lugea A, Kolodecik TR, Yuan J, Reeve JR Jr, Gorelick FS, Pandol SJ. Protein kinase C delta-mediated processes in cholecystokinin-8-stimulated pancreatic acini. Pancreas 38: 930–935, 2009. doi: 10.1097/MPA.0b013e3181b8476a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tyagi N, Bhardwaj A, Singh AP, McClellan S, Carter JE, Singh S. p-21 activated kinase 4 promotes proliferation and survival of pancreatic cancer cells through AKT- and ERK-dependent activation of NF-κB pathway. Oncotarget 5: 8778–8789, 2014. doi: 10.18632/oncotarget.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Voronina S, Collier D, Chvanov M, Middlehurst B, Beckett AJ, Prior IA, Criddle DN, Begg M, Mikoshiba K, Sutton R, Tepikin AV. The role of Ca2+ influx in endocytic vacuole formation in pancreatic acinar cells. Biochem J 465: 405–412, 2015. doi: 10.1042/BJ20140398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wallace SW, Durgan J, Jin D, Hall A. Cdc42 regulates apical junction formation in human bronchial epithelial cells through PAK4 and Par6B. Mol Biol Cell 21: 2996–3006, 2010. doi: 10.1091/mbc.e10-05-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang C, Li Y, Zhang H, Liu F, Cheng Z, Wang D, Wang G, Xu H, Zhao Y, Cao L, Li F. Oncogenic PAK4 regulates Smad2/3 axis involving gastric tumorigenesis. Oncogene 33: 3473–3484, 2014. doi: 10.1038/onc.2013.300. [DOI] [PubMed] [Google Scholar]
- 77.Wang LH, Coy DH, Taylor JE, Jiang NY, Moreau JP, Huang SC, Frucht H, Haffar BM, Jensen RT. des-Met carboxyl-terminally modified analogues of bombesin function as potent bombesin receptor antagonists, partial agonists, or agonists. J Biol Chem 265: 15695–15703, 1990. [PubMed] [Google Scholar]
- 78.Wang Z, Zhang X, Yang Z, Du H, Wu Z, Gong J, Yan J, Zheng Q. MiR-145 regulates PAK4 via the MAPK pathway and exhibits an antitumor effect in human colon cells. Biochem Biophys Res Commun 427: 444–449, 2012. doi: 10.1016/j.bbrc.2012.06.123. [DOI] [PubMed] [Google Scholar]
- 79.Weber HC. Regulation and signaling of human bombesin receptors and their biological effects. Curr Opin Endocrinol Diabetes Obes 16: 66–71, 2009. doi: 10.1097/MED.0b013e32831cf5aa. [DOI] [PubMed] [Google Scholar]
- 80.Wells CM, Abo A, Ridley AJ. PAK4 is activated via PI3K in HGF-stimulated epithelial cells. J Cell Sci 115: 3947–3956, 2002. doi: 10.1242/jcs.00080. [DOI] [PubMed] [Google Scholar]
- 81.Wong LL, Lam IP, Wong TY, Lai WL, Liu HF, Yeung LL, Ching YP. IPA-3 inhibits the growth of liver cancer cells by suppressing PAK1 and NF-κB activation. PLoS One 8: e68843, 2013. doi: 10.1371/journal.pone.0068843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xie X, Shi X, Guan H, Guo Q, Fan C, Dong W, Wang G, Li F, Shan Z, Cao L, Teng W. P21-activated kinase 4 involves TSH induced papillary thyroid cancer cell proliferation. Oncotarget 8: 24882–24891, 2017. doi: 10.18632/oncotarget.15079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ye DZ, Field J. PAK signaling in cancer. Cell Logist 2: 105–116, 2012. doi: 10.4161/cl.21882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yeo D, He H, Baldwin GS, Nikfarjam M. The role of p21-activated kinases in pancreatic cancer. Pancreas 44: 363–369, 2015. doi: 10.1097/MPA.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 85.Yoder SM, Dineen SL, Wang Z, Thurmond DC. YES, a Src family kinase, is a proximal glucose-specific activator of cell division cycle control protein 42 (Cdc42) in pancreatic islet β cells. J Biol Chem 289: 11476–11487, 2014. doi: 10.1074/jbc.M114.559328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yun CY, You ST, Kim JH, Chung JH, Han SB, Shin EY, Kim EG. p21-activated kinase 4 critically regulates melanogenesis via activation of the CREB/MITF and β-catenin/MITF pathways. J Invest Dermatol 135: 1385–1394, 2015. doi: 10.1038/jid.2014.548. [DOI] [PubMed] [Google Scholar]
- 87.Zhang J, Wang J, Guo Q, Wang Y, Zhou Y, Peng H, Cheng M, Zhao D, Li F. LCH-7749944, a novel and potent p21-activated kinase 4 inhibitor, suppresses proliferation and invasion in human gastric cancer cells. Cancer Lett 317: 24–32, 2012. [Erratum in Cancer Lett 349: 159, 2014.] doi: 10.1016/j.canlet.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 88.Zhao M, Rabieifar P, Costa TDF, Zhuang T, Minden A, Löhr M, Heuchel R, Strömblad S. Pdx1-Cre-driven conditional gene depletion suggests PAK4 as dispensable for mouse pancreas development. Sci Rep 7: 7031, 2017. doi: 10.1038/s41598-017-07322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao ZS, Manser E. PAK family kinases: physiological roles and regulation. Cell Logist 2: 59–68, 2012. doi: 10.4161/cl.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]